Integrate lanes from all batches of EB pilot (Human data only, no doublets
Last updated: 2020-08-10
Checks: 7 0
Knit directory: Embryoid_Body_Pilot_Workflowr/analysis/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20200804) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version f50ebd3. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: output/.Rhistory
Untracked files:
Untracked: analysis/child/
Untracked: code/EB.getHumanMetadata.Rmd
Untracked: figure/
Untracked: output/mergedObjects/
Untracked: output/sampleQCrds/
Unstaged changes:
Modified: .Rprofile
Modified: .gitattributes
Modified: .gitignore
Modified: Embryoid_Body_Pilot_Workflowr.Rproj
Modified: README.md
Modified: _workflowr.yml
Modified: analysis/_site.yml
Modified: analysis/about.Rmd
Modified: analysis/index.Rmd
Modified: analysis/license.Rmd
Modified: code/README.md
Modified: data/README.md
Modified: output/README.md
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/IntegrateAnalysis.afterFilter.HarmonyBatchSampleIDindividual.Rmd) and HTML (docs/IntegrateAnalysis.afterFilter.HarmonyBatchSampleIDindividual.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| html | 421a225 | KLRhodes | 2020-08-10 | Build site. |
| Rmd | bc8ec6f | KLRhodes | 2020-08-10 | cleaning various versions of merging/intCurrent working directory |
library(Seurat)
library(harmony)
library(ggplot2)
library(DataCombine)
library(here)
library(RColorBrewer)
options(future.globals.maxSize= 15000*1024^2) #allow global exceeding 4GbRead in the files, add metadata, and create an object list
filelist<-list.files(here::here('output/sampleQCrds/'), full.names = T)
objectlist<- list()
for (i in 1:length(filelist)){
rds<- readRDS(filelist[i])
objectlist[i]<- rds
}Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecated
Warning in `[<-`(`*tmp*`, i, value = rds): implicit list embedding of S4 objects
is deprecatedcreate a merged seurat object
ids<-substr(basename(filelist),1,12)
merged<- merge(objectlist[[1]], c(objectlist[[2]], objectlist[[3]],objectlist[[4]],objectlist[[5]],objectlist[[6]],objectlist[[7]],objectlist[[8]],objectlist[[9]],objectlist[[10]],objectlist[[11]],objectlist[[12]],objectlist[[13]],objectlist[[14]],objectlist[[15]],objectlist[[16]]),add.cell.ids=ids, merge.data=T)#need to fix the individual names because they are slightly different from batch1
replacements<- data.frame(from= c("SNG-NA18511.variant2", "SNG-NA18858.variant2", "SNG-NA19160.variant2"), to=c("SNG-NA18511", "SNG-NA18858", "SNG-NA19160"))
merged@meta.data<-FindReplace(merged@meta.data, "individual", replacements, from = "from", to= "to", exact=T, vector=F )Only exact matches will be replaced.#run PCA on full dataset pre-alignment
all.genes= rownames(merged)
merged<-FindVariableFeatures(merged,selection.method="vst", nfeatures = 5000)
#have previously used all genes (nfeatures=25000) and clustering by individual rather than batch (based on proportion of cells per cluster) was still observed downstream. Now using 5000 because it is the upper bound of what has been recommended in the literature.
merged<- ScaleData(merged, features = all.genes)Centering and scaling data matrixmerged<-RunPCA(merged, npcs = 100, verbose=F)DimPlot(merged, reduction = "pca", group.by = "Batch")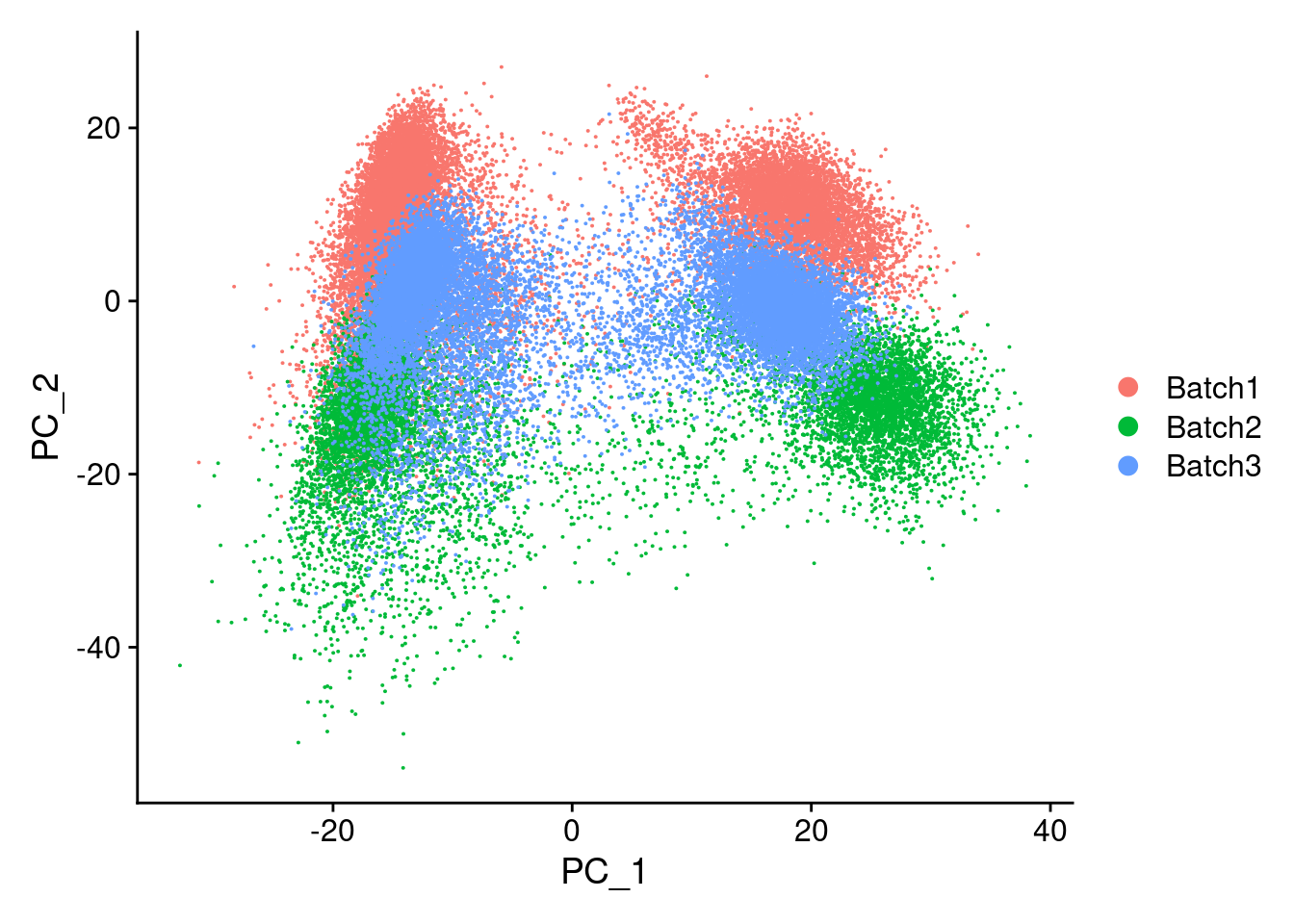
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
Now, running harmony to integrate. Here, using Batch, SampleID(10x Lane), and individual to integrate. Since Batch and Lane are confounded, this may over correct for Batch.
merged<- RunHarmony(merged, c("Batch", "SampleID", "individual"), plot_convergence = T, assay.use = "SCT")Harmony 1/10Harmony 2/10Harmony 3/10Harmony 4/10Harmony 5/10Harmony 6/10Harmony 7/10Harmony converged after 7 iterationsWarning: Invalid name supplied, making object name syntactically valid. New
object name is Seurat..ProjectDim.SCT.harmony; see ?make.names for more details
on syntax validity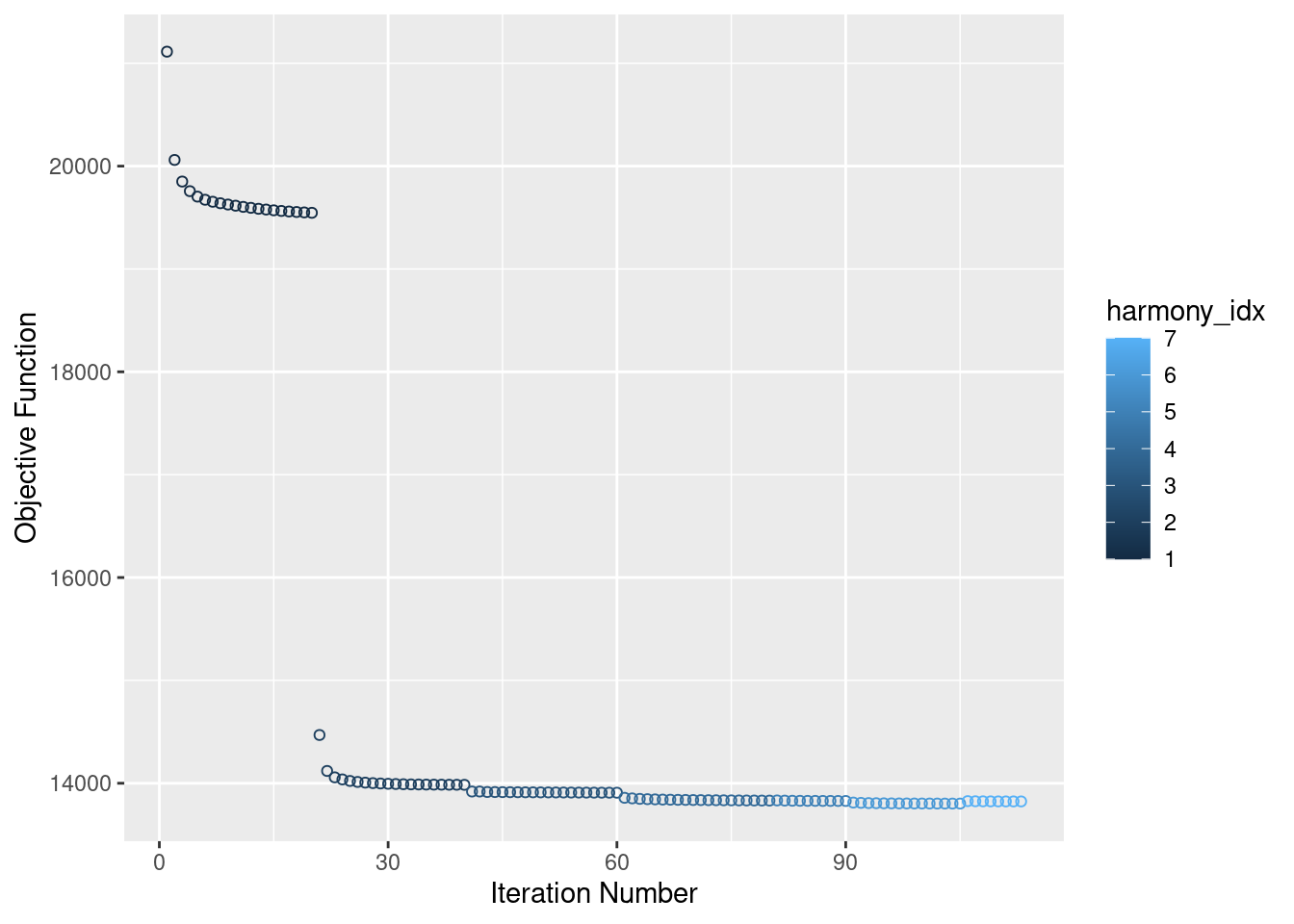
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
Visualize Harmony embeddings
DimPlot(merged, reduction="harmony", group.by= c("individual", "Batch"), combine=F)[[1]]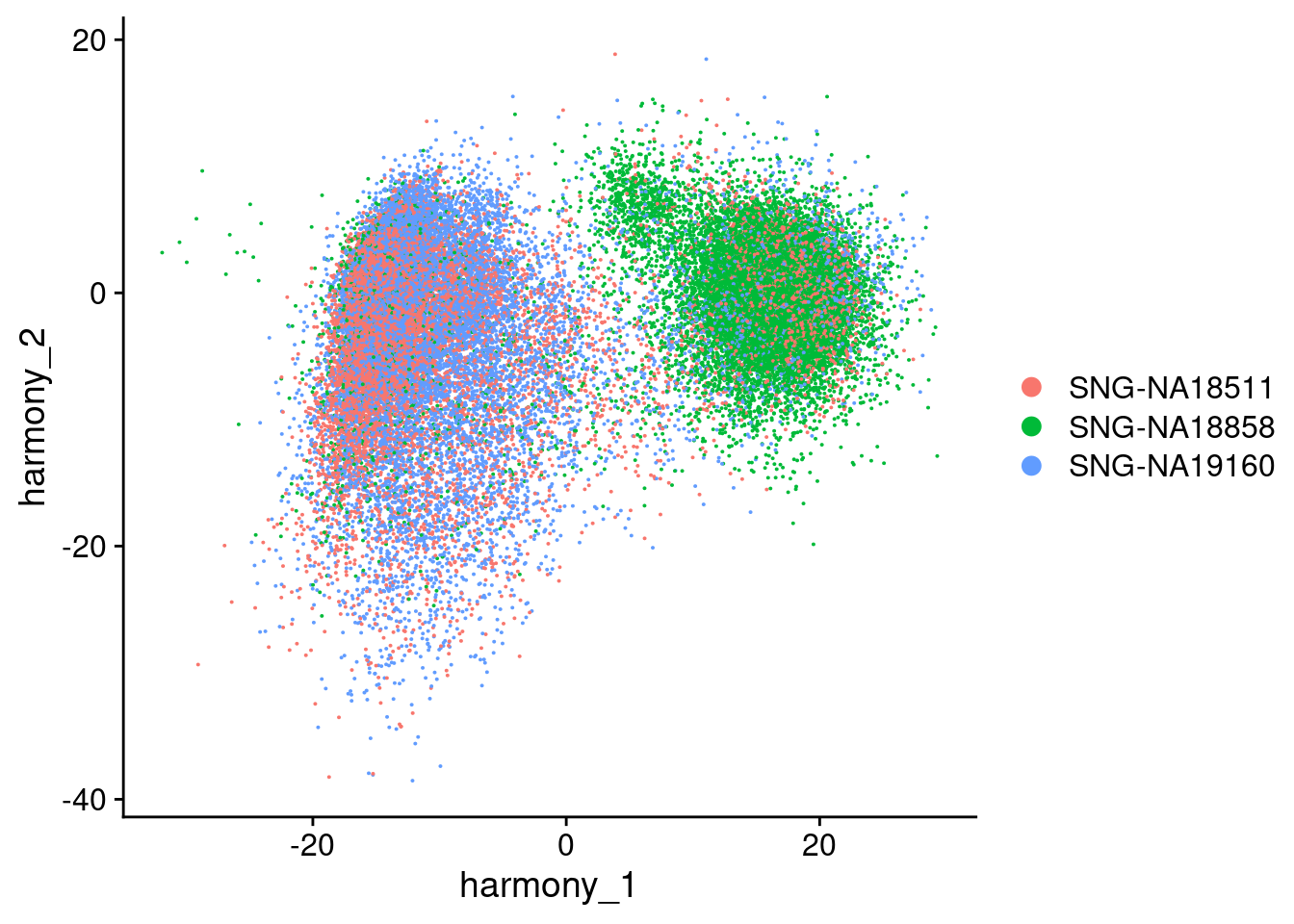
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[2]]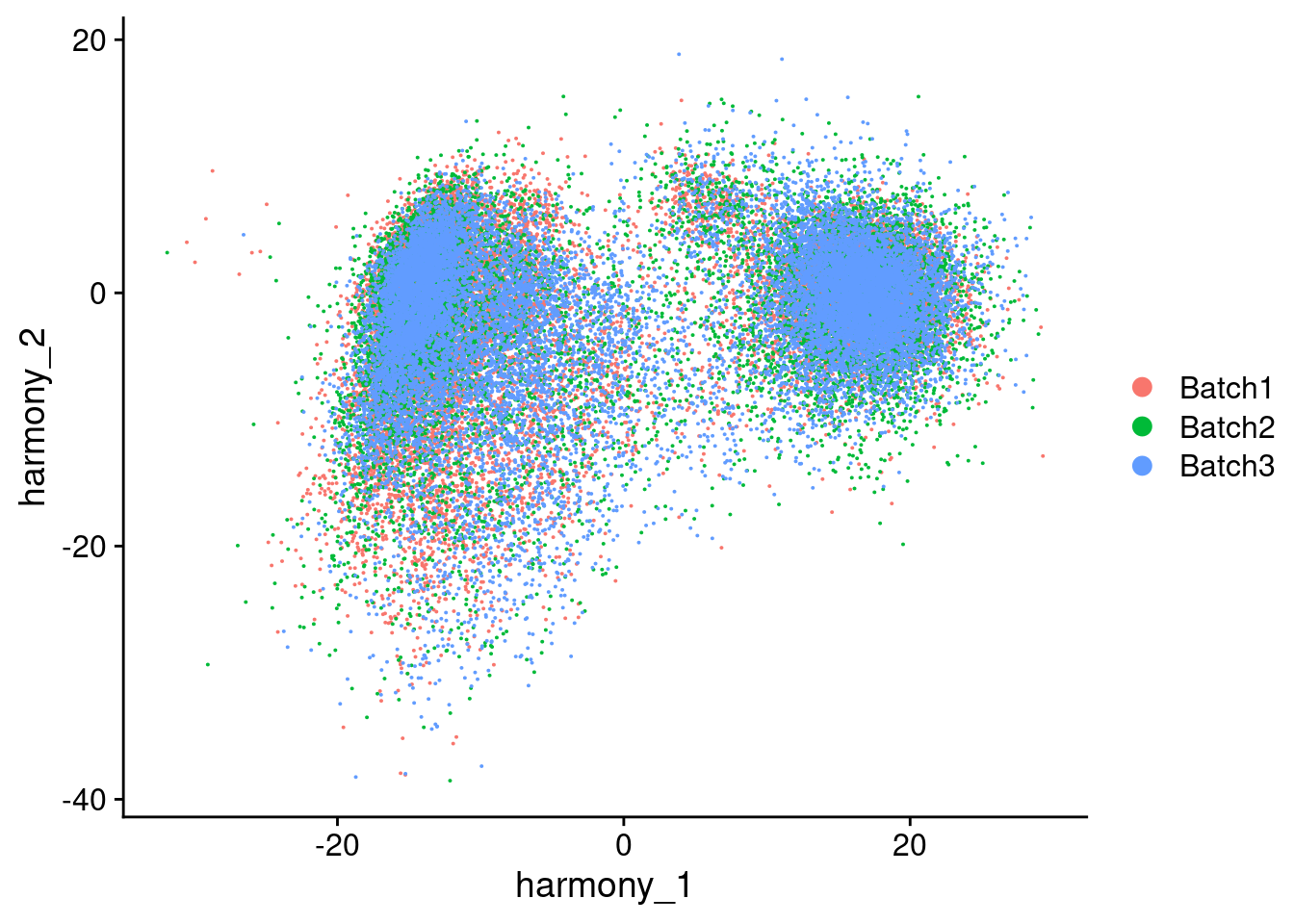
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
Now Running UMAP and identifying clusters, etc
merged<- RunUMAP(merged, reduction = "harmony", dims = 1:100, verbose = F)Warning: The default method for RunUMAP has changed from calling Python UMAP via reticulate to the R-native UWOT using the cosine metric
To use Python UMAP via reticulate, set umap.method to 'umap-learn' and metric to 'correlation'
This message will be shown once per sessionmerged<- FindNeighbors(merged, reduction="harmony", dims = 1:100, verbose = F)
merged<- FindClusters(merged, resolution=1, verbose = F)
merged<- FindClusters(merged, resolution=0.8, verbose = F)
merged<- FindClusters(merged, resolution=0.5, verbose = F)
merged<- FindClusters(merged, resolution=0.1, verbose = F)SAVING merged/aligned/reclustered object
path<- here::here("output/mergedObjects/")
saveRDS(merged, file=paste0(path,'Harmony.BatchSampleIDindividual.rds'))#reassign idents
Idents(merged)<- 'SCT_snn_res.1'VizDimLoadings(merged, dims = 1:2, reduction = "harmony")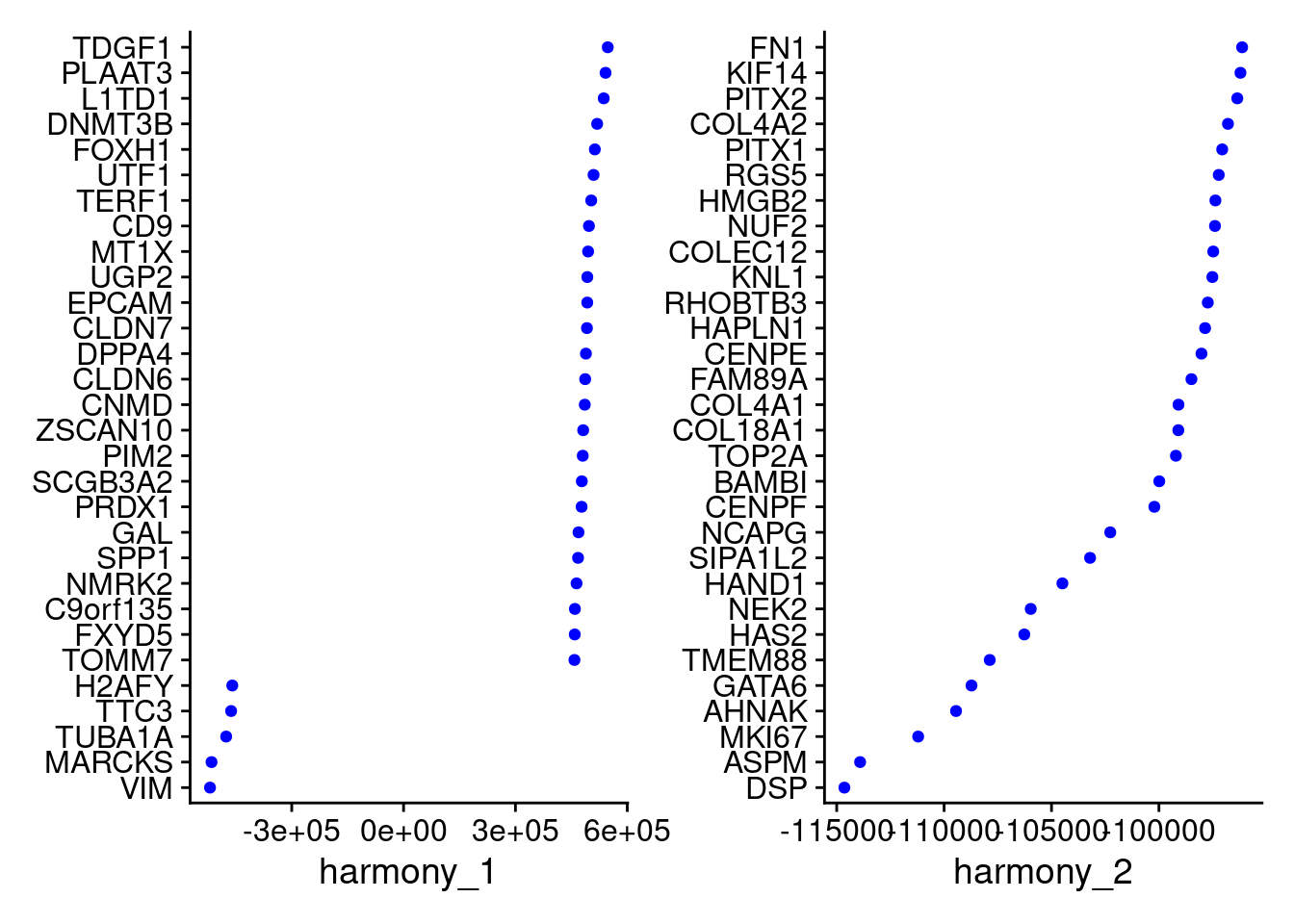
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
VizDimLoadings(merged, dims = 3:4, reduction = "harmony")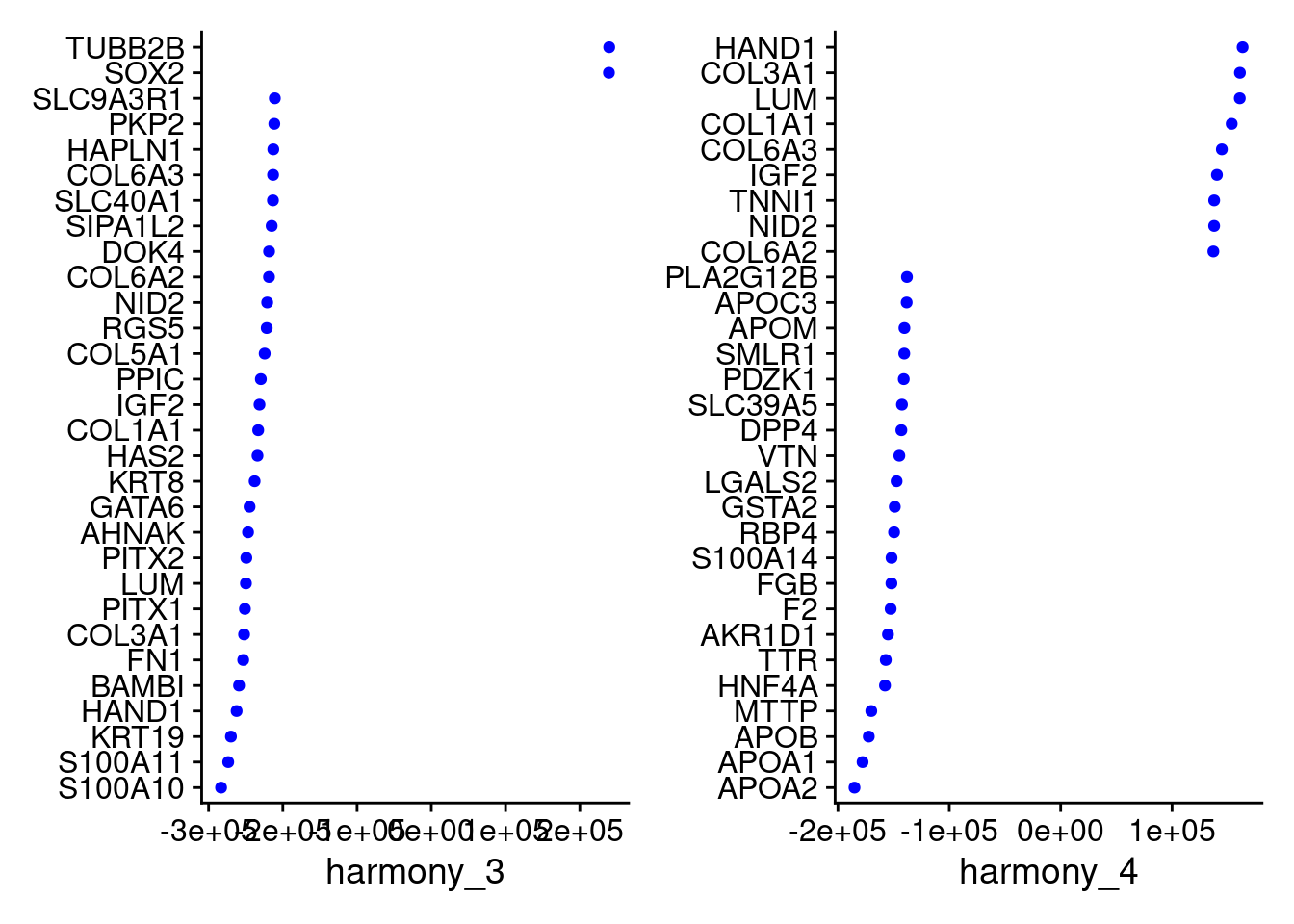
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
VizDimLoadings(merged, dims = 5:6, reduction = "harmony")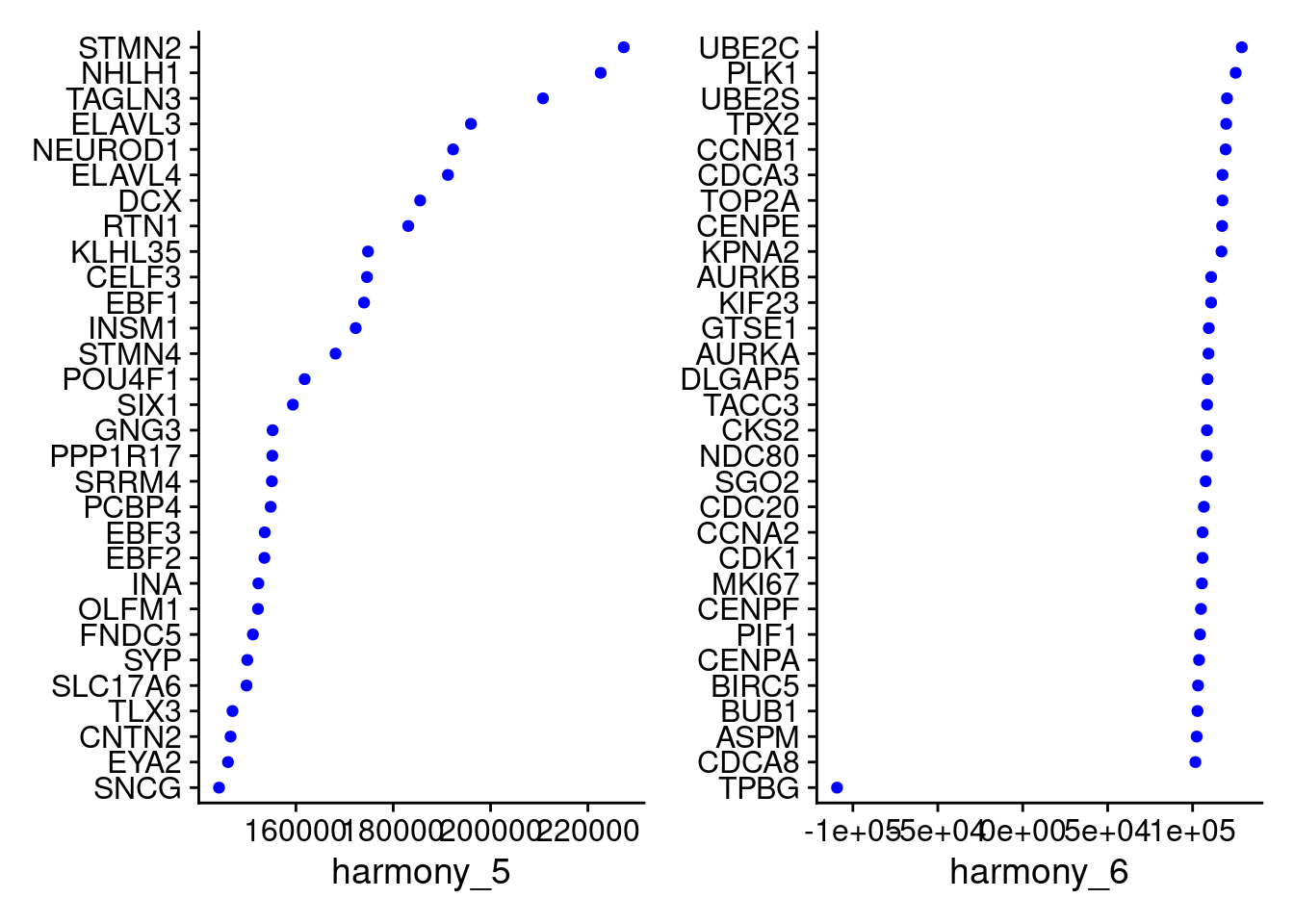
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
xlim <- c(min(merged@reductions$harmony@cell.embeddings[,'harmony_1']),
max(merged@reductions$harmony@cell.embeddings[,'harmony_1']))
ylim <- c(min(merged@reductions$harmony@cell.embeddings[,'harmony_2']),
max(merged@reductions$harmony@cell.embeddings[,'harmony_2']))
individuals <- table(merged$individual)
individuals <- individuals[individuals>50]
individuals <- names(individuals)
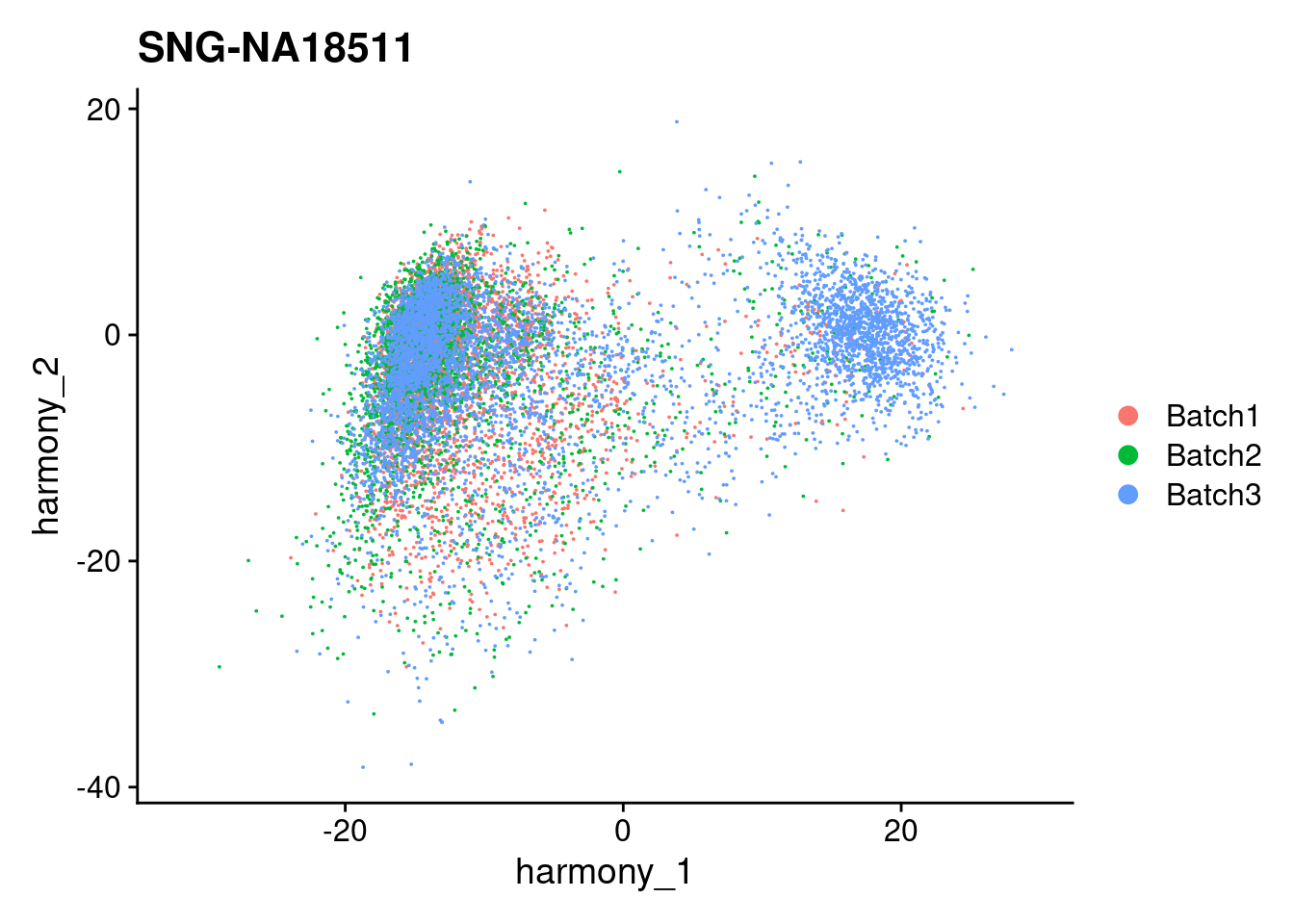
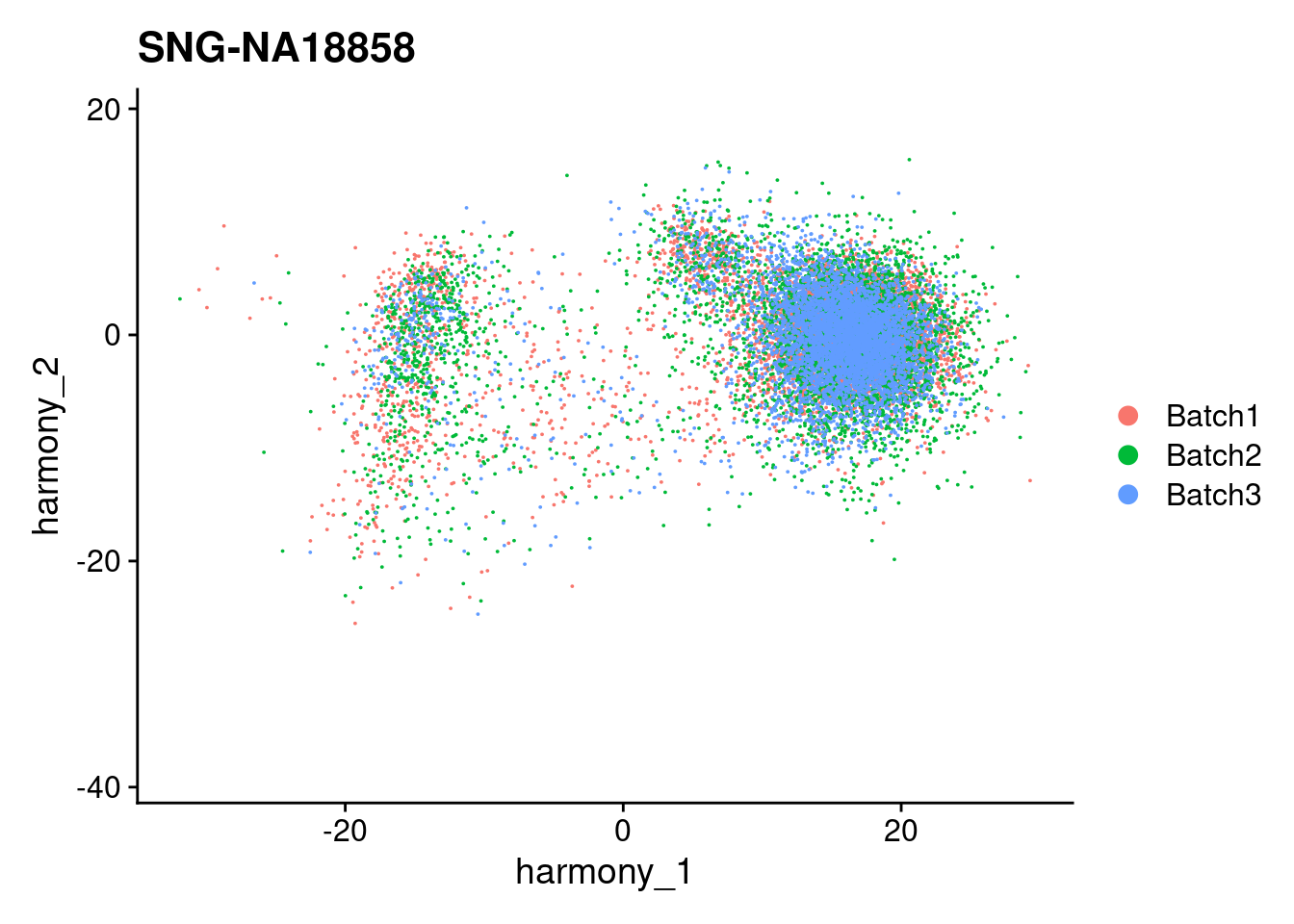
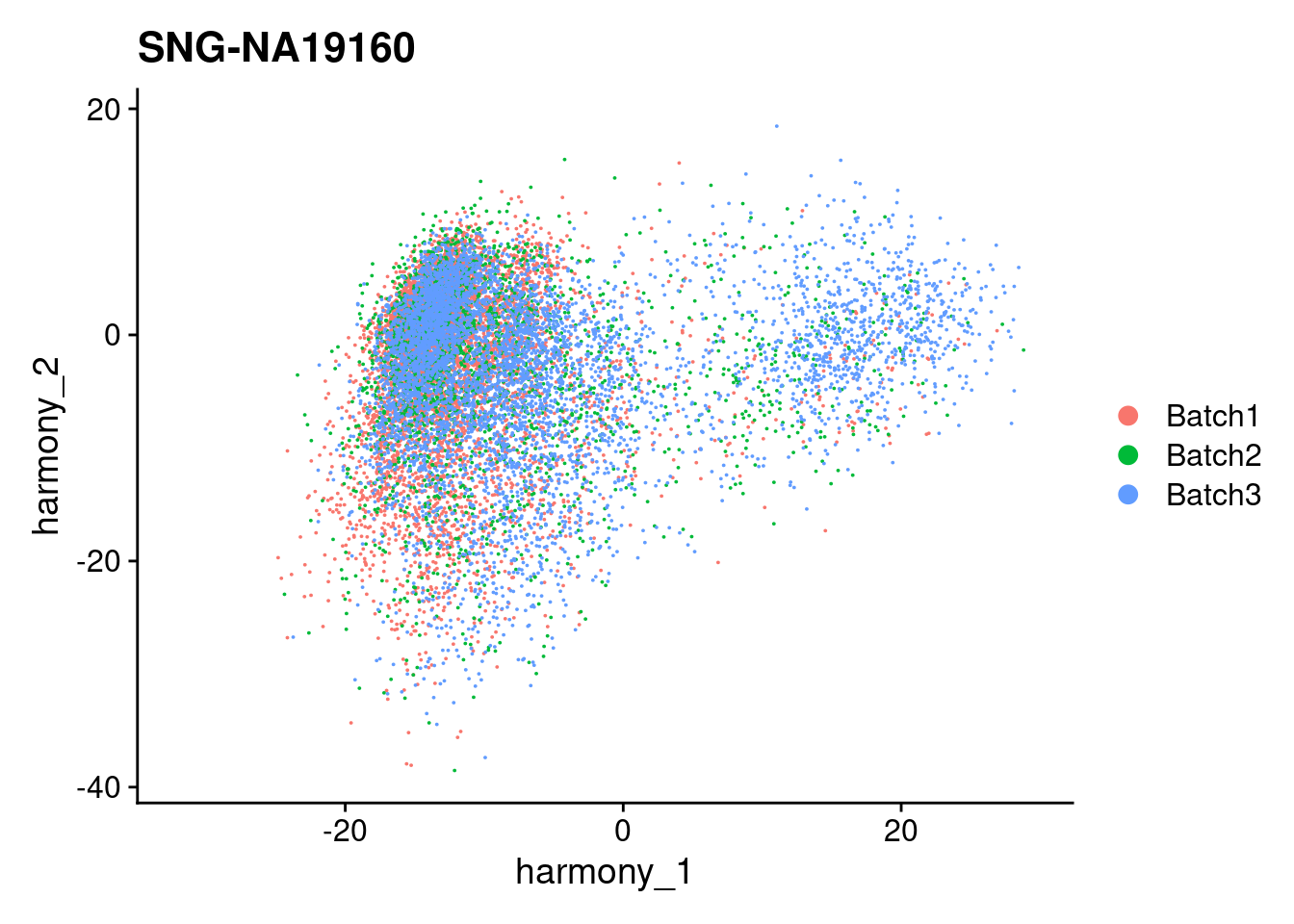
for (i in individuals)
{
print(DimPlot(merged, reduction = "harmony", group.by = c("Batch"), pt.size = 0.01,
cells = WhichCells(merged, expression = individual == i)) +
xlim(xlim) + ylim(ylim) + ggtitle(i))
}
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap")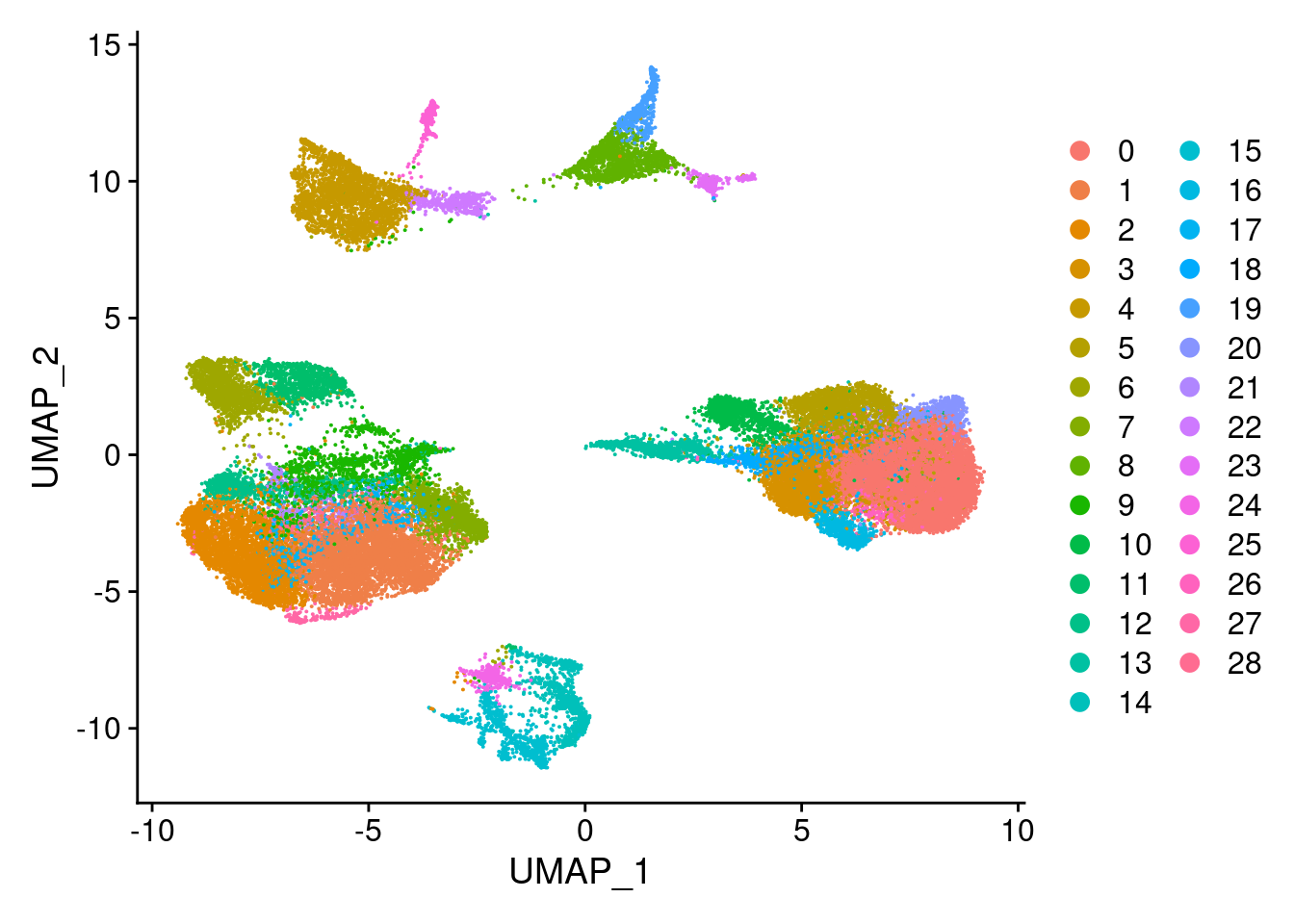
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap", group.by = "Batch")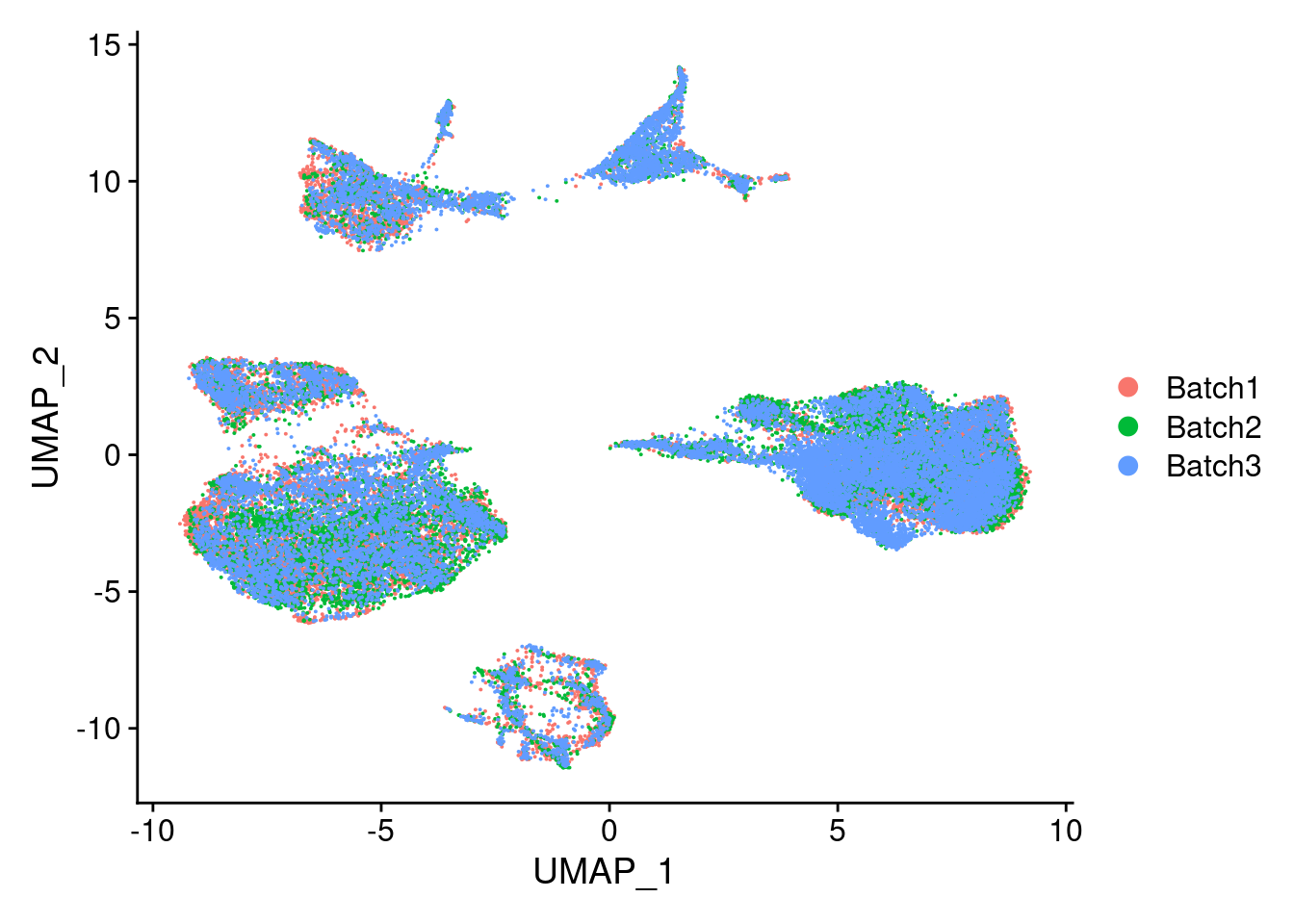
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap", group.by = "individual")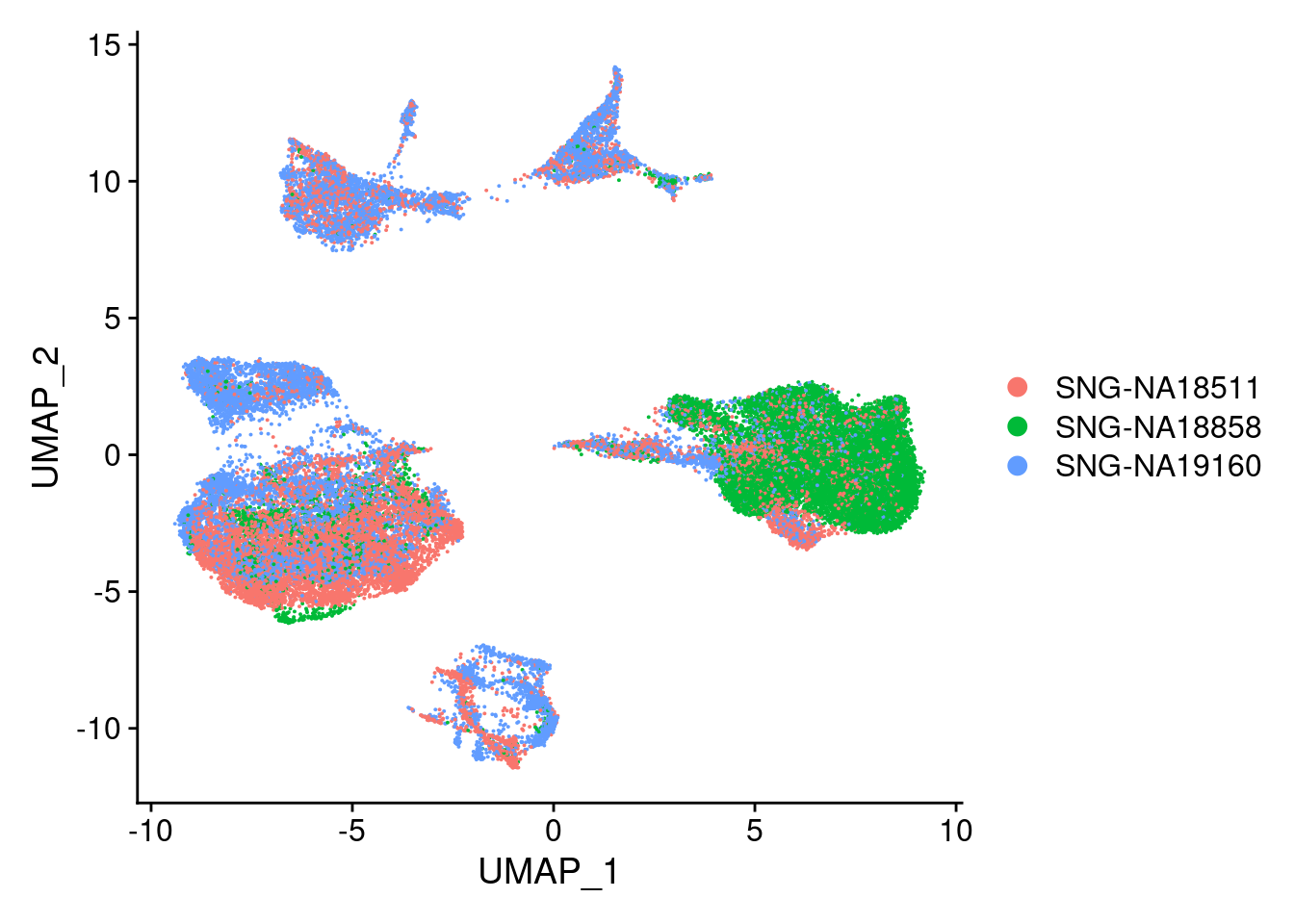
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
xlim <- c(min(merged@reductions$umap@cell.embeddings[,'UMAP_1']),
max(merged@reductions$umap@cell.embeddings[,'UMAP_1']))
ylim <- c(min(merged@reductions$umap@cell.embeddings[,'UMAP_2']),
max(merged@reductions$umap@cell.embeddings[,'UMAP_2']))
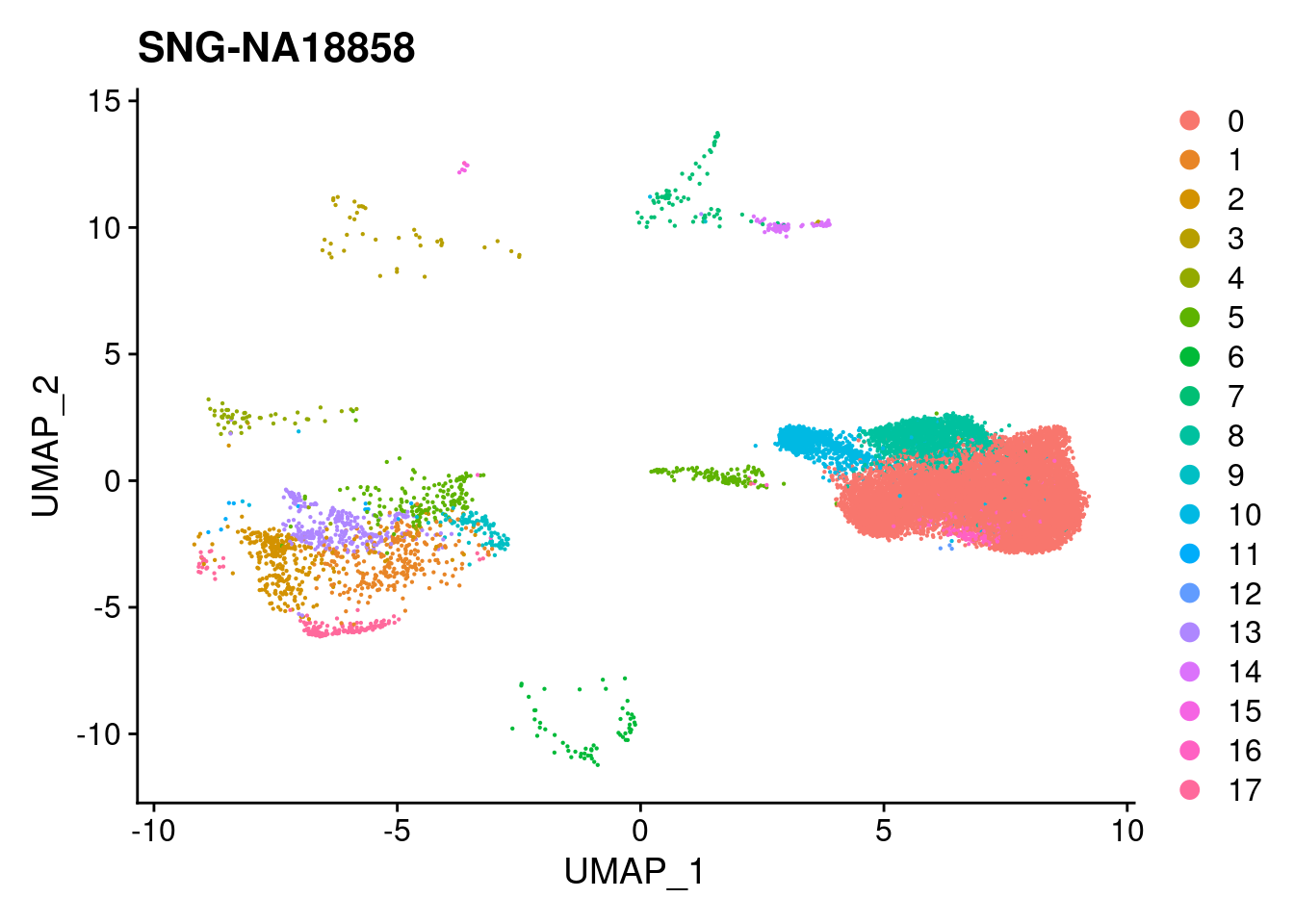
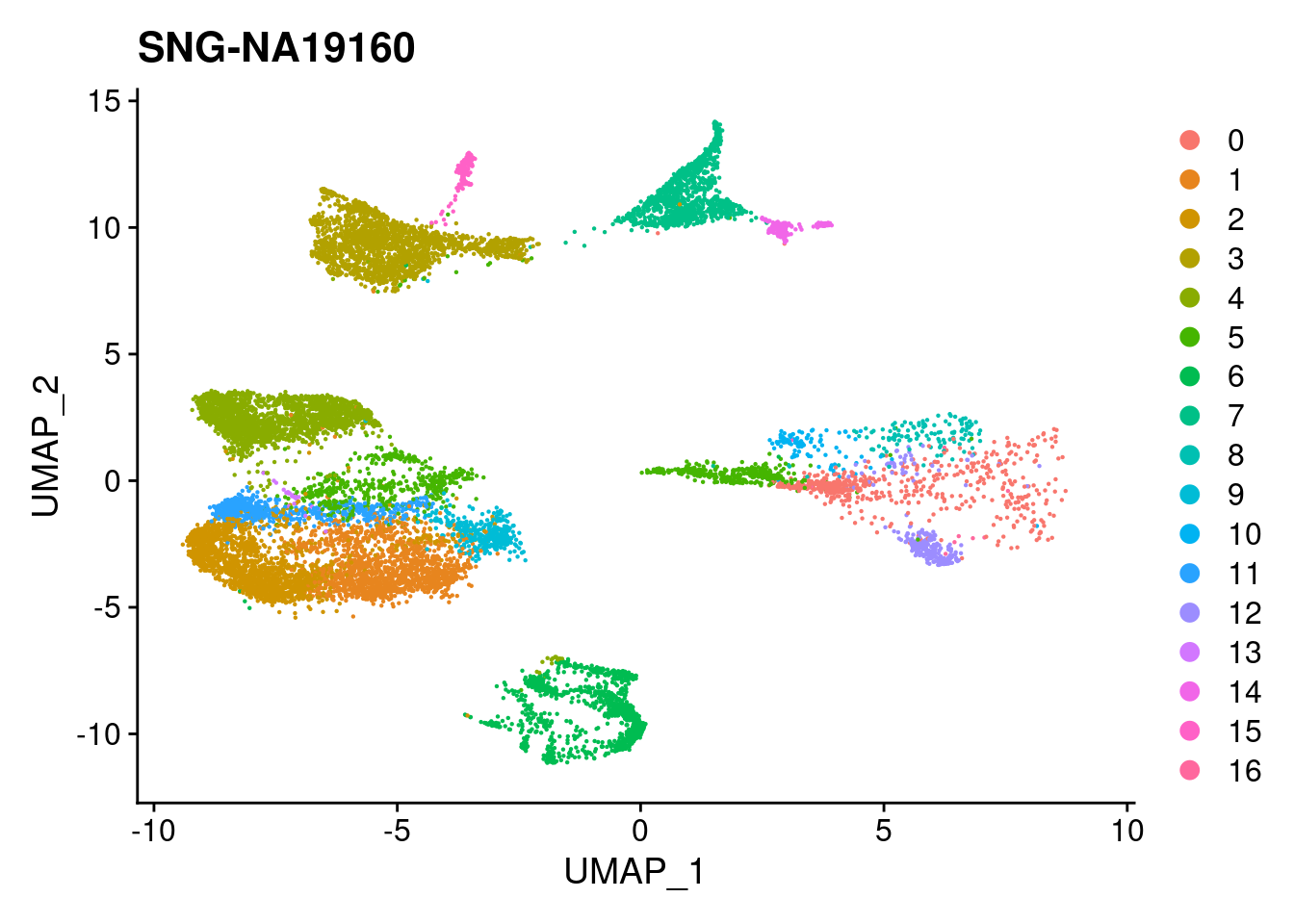
for (i in individuals)
{
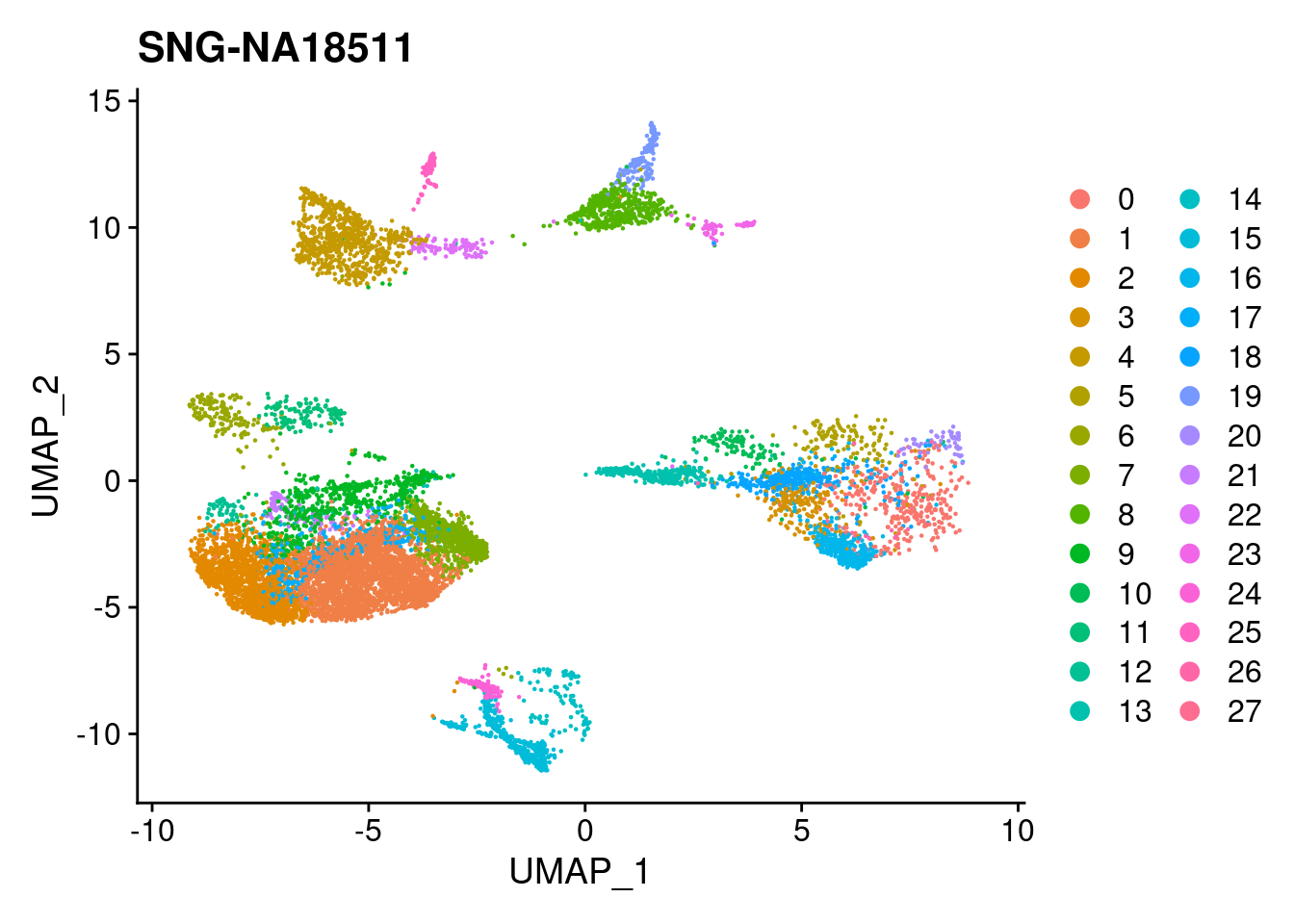
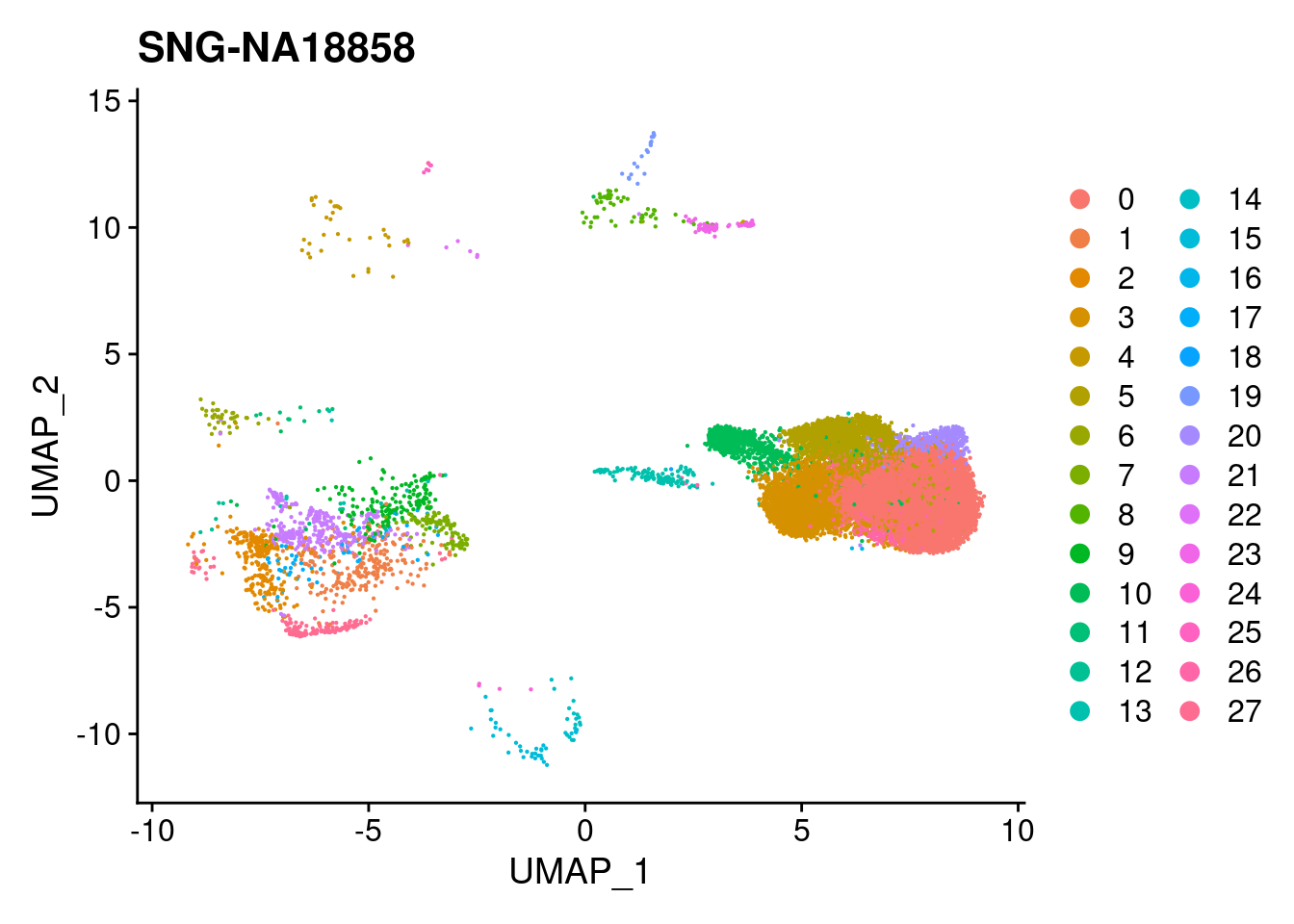
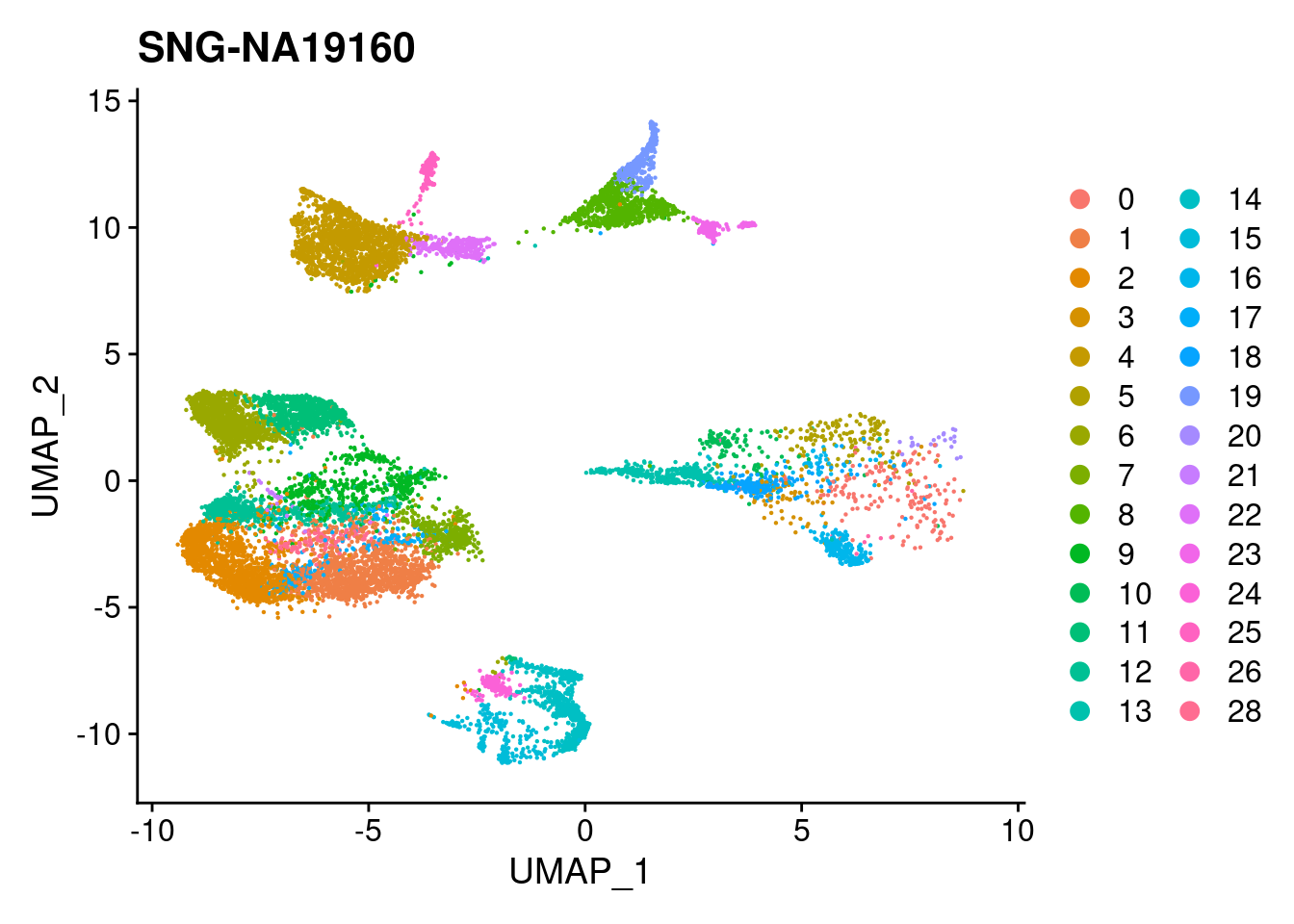
print(DimPlot(merged, reduction = "umap",
cells = WhichCells(merged, expression = individual == i)) +
xlim(xlim) + ylim(ylim) + ggtitle(i))
}
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
plots2<- DimPlot(merged, group.by = "individual", split.by = "Batch")
plots2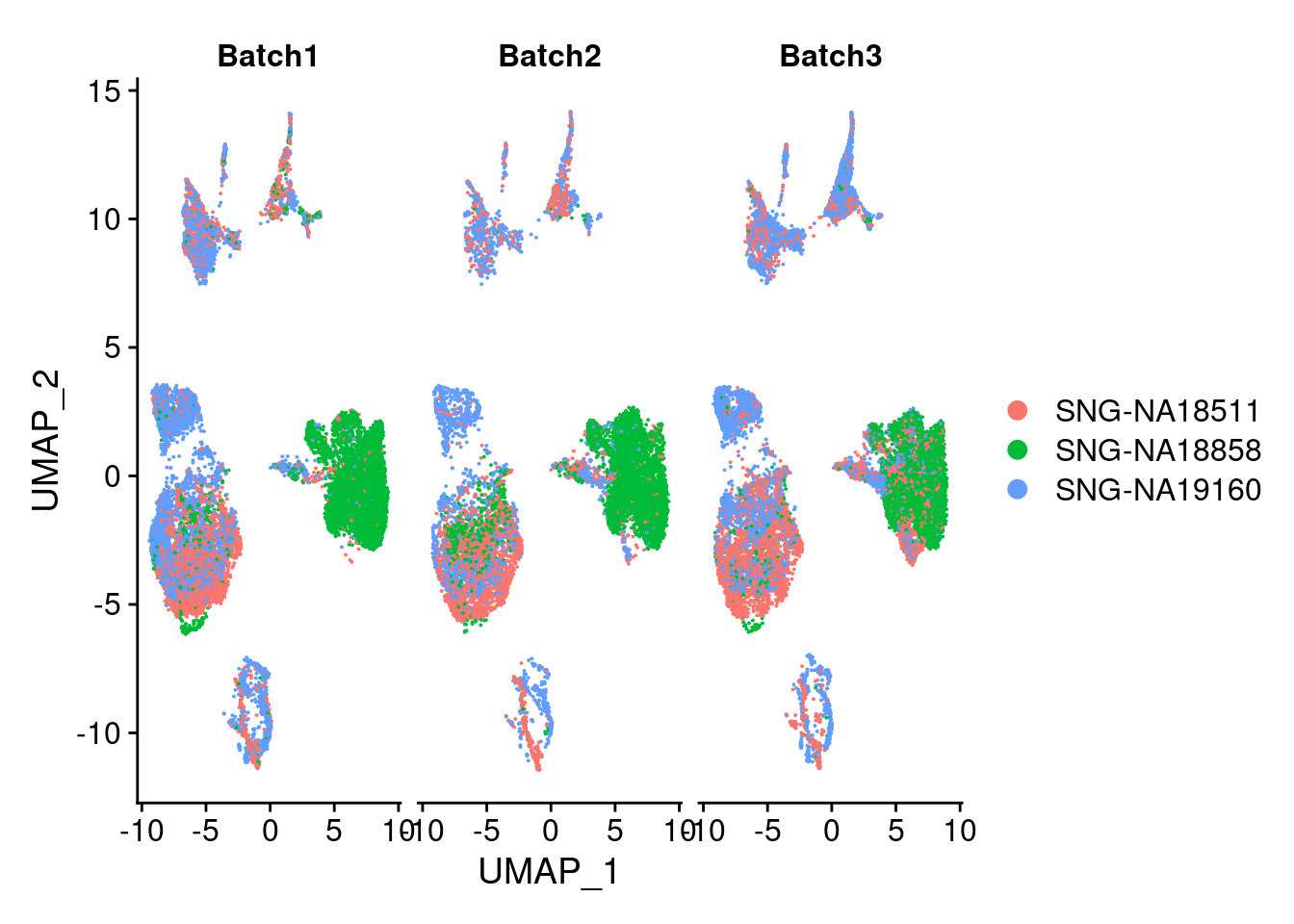
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, group.by = "Batch", split.by = c("individual"))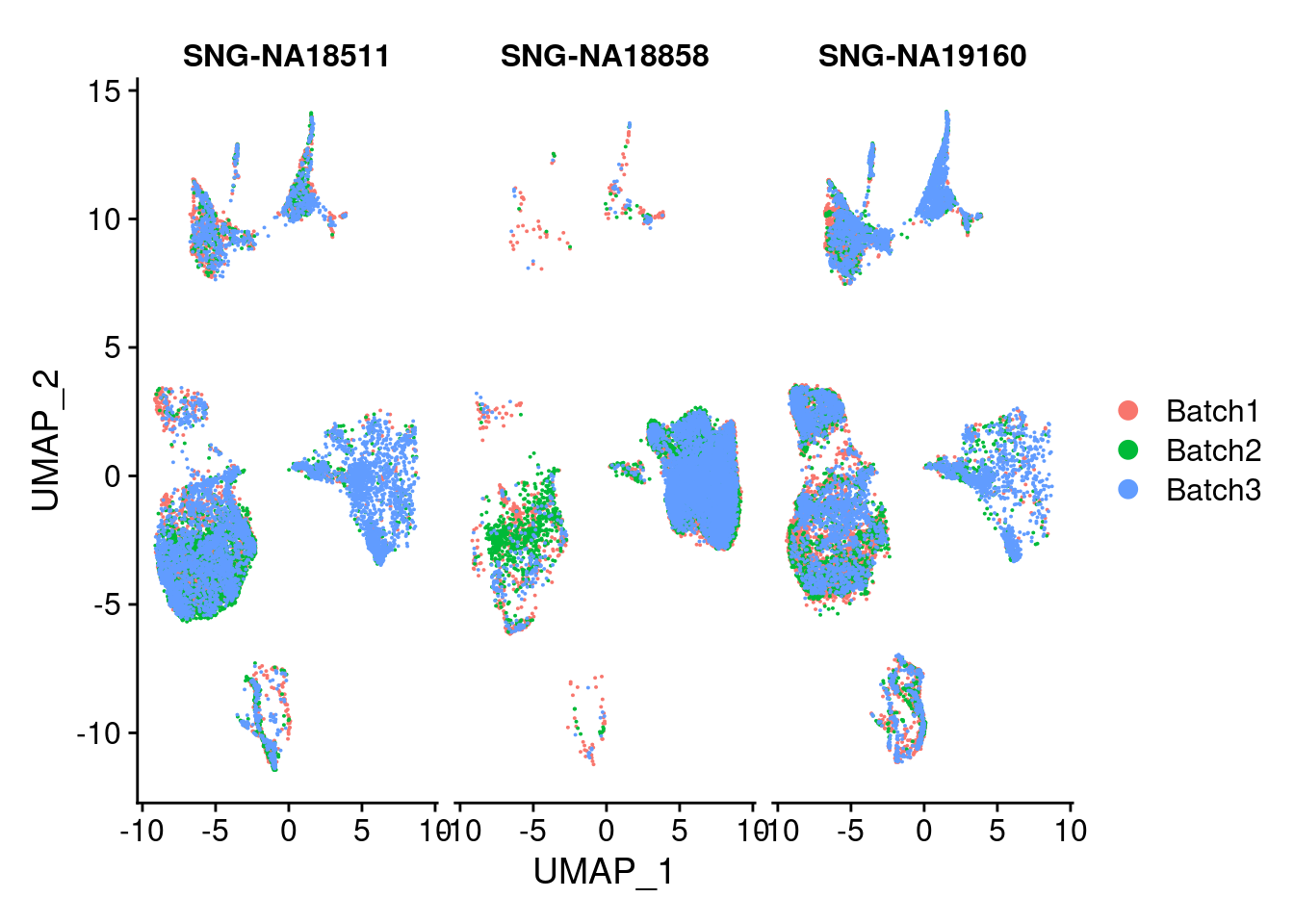
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, group.by = "SCT_snn_res.1", split.by = c("Batch"), label=T)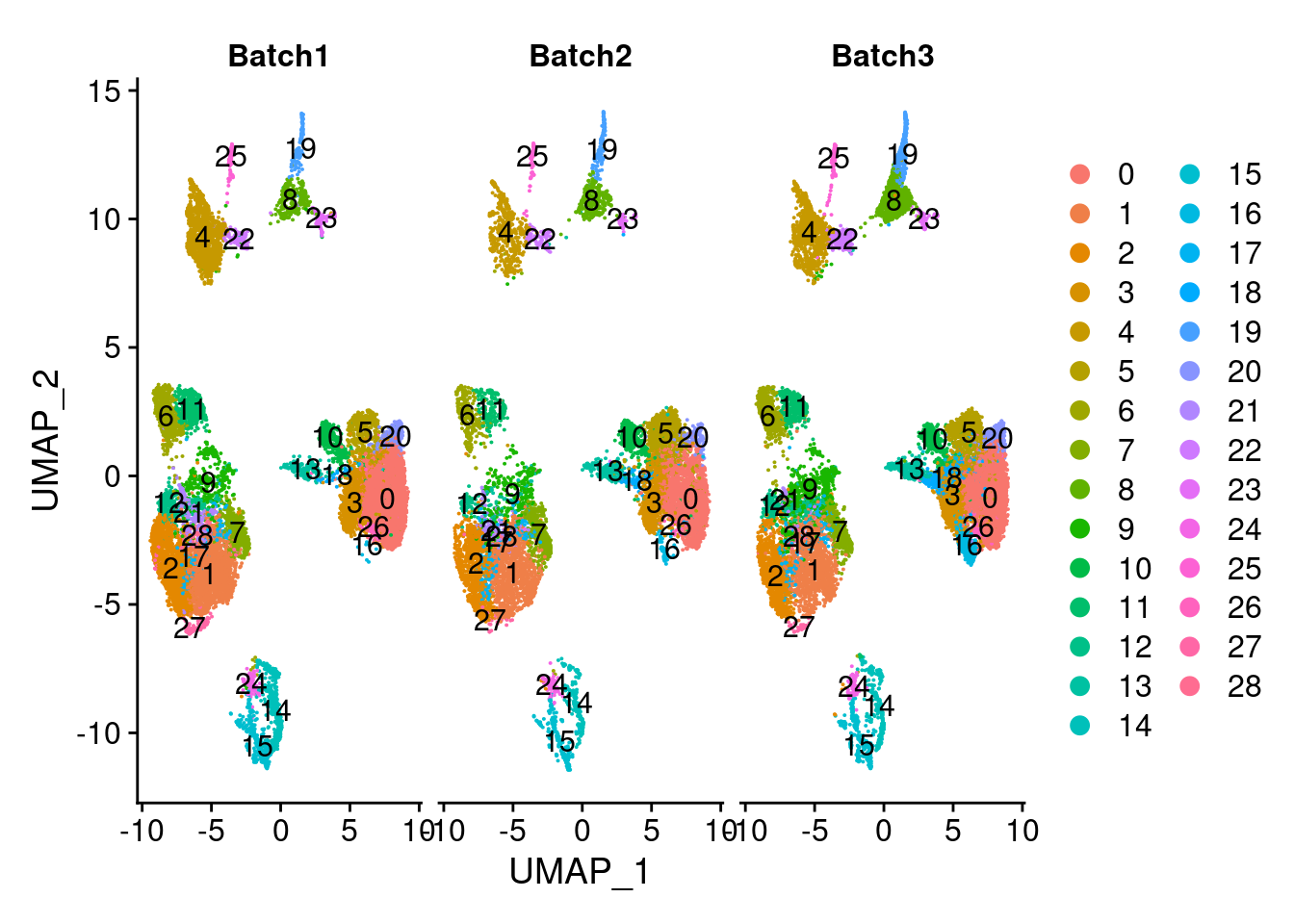
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "harmony", group.by = "SCT_snn_res.1", split.by = "Batch", combine = F)[[1]]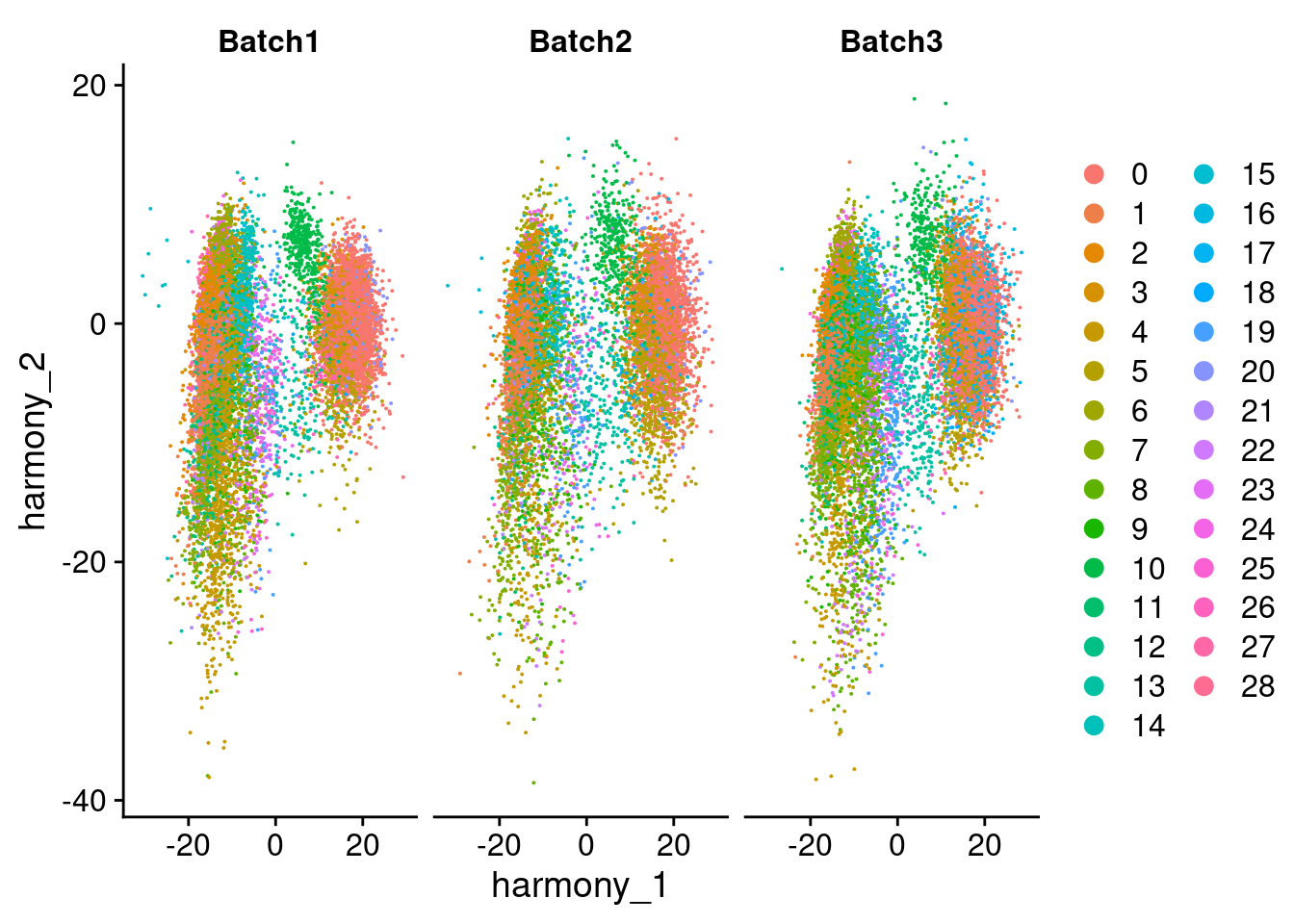
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
VlnPlot(merged, features = c("POU5F1", "PAX6", "TNNT2", "SOX17", "HAND1", "LUM"), ncol=2)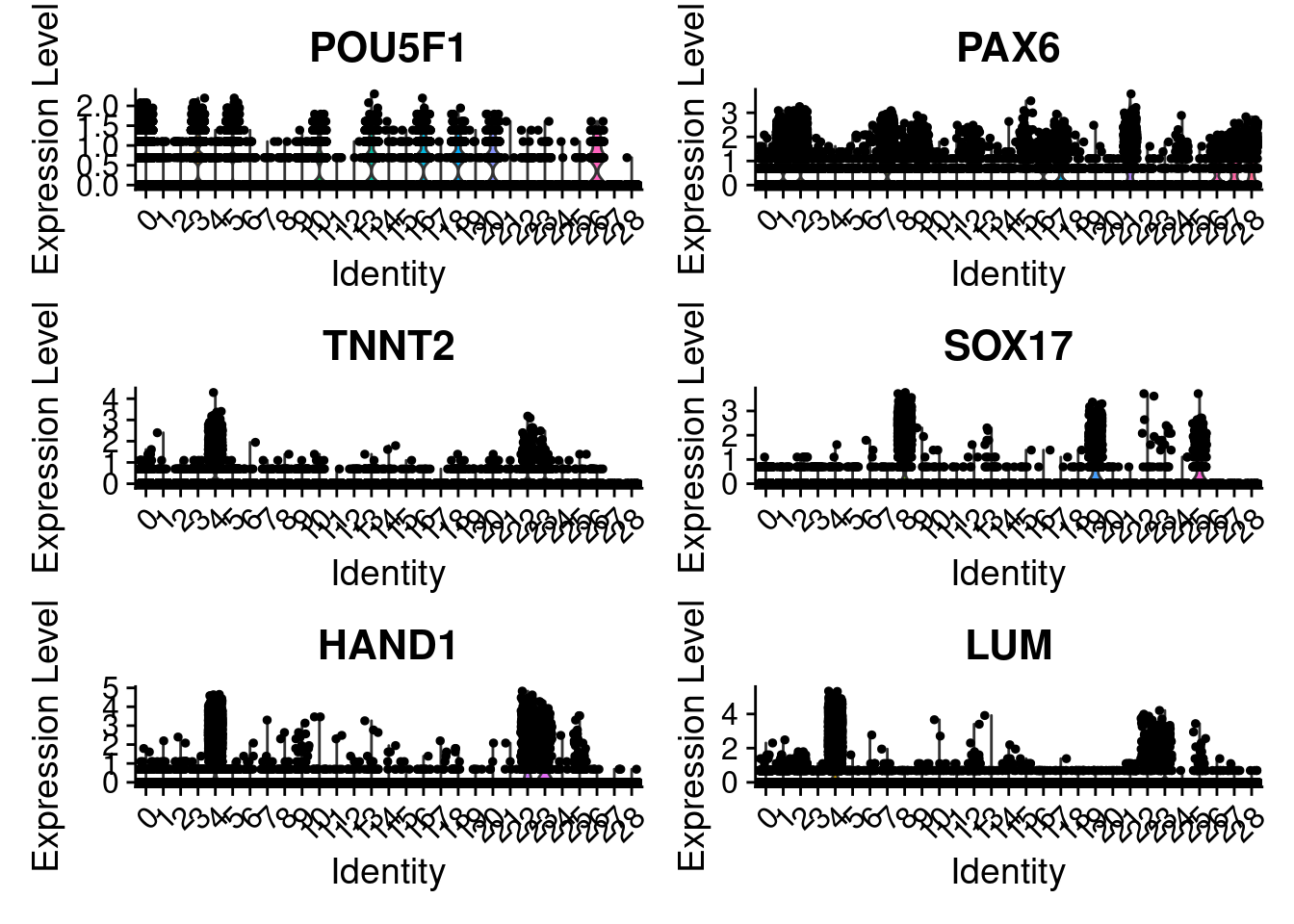
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#pluripotent markers
FeaturePlot(merged, features = c("POU5F1", "SOX2", "NANOG"), pt.size = 0.2, ncol=3)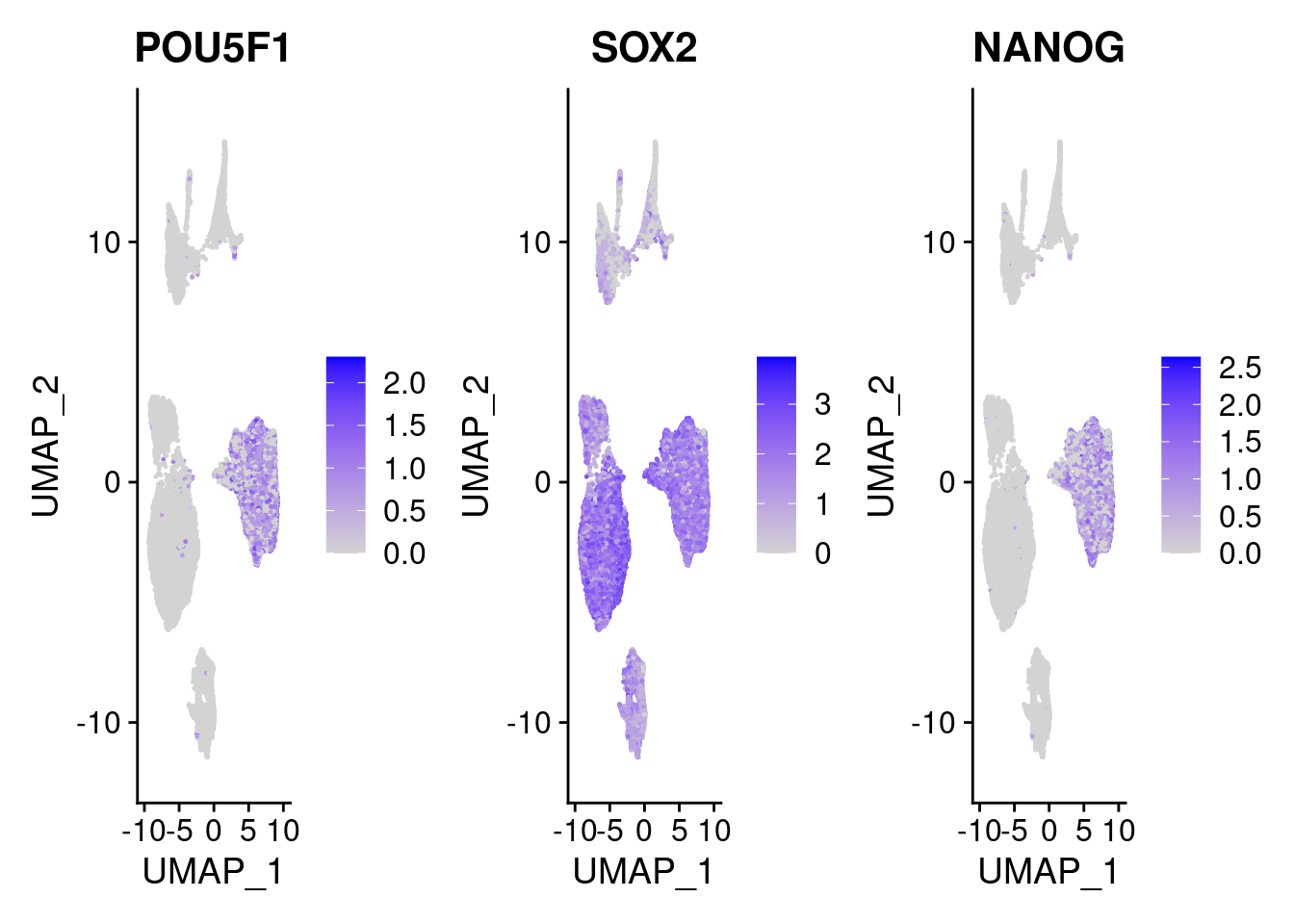
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#Endoderm markers (first 3 definitive endo, 4-6 liver markers, )
FeaturePlot(merged, features = c("SOX17","CLDN6","FOXA2", "TTR", "AFP", "FGB"), pt.size = 0.2, combine = F)[[1]]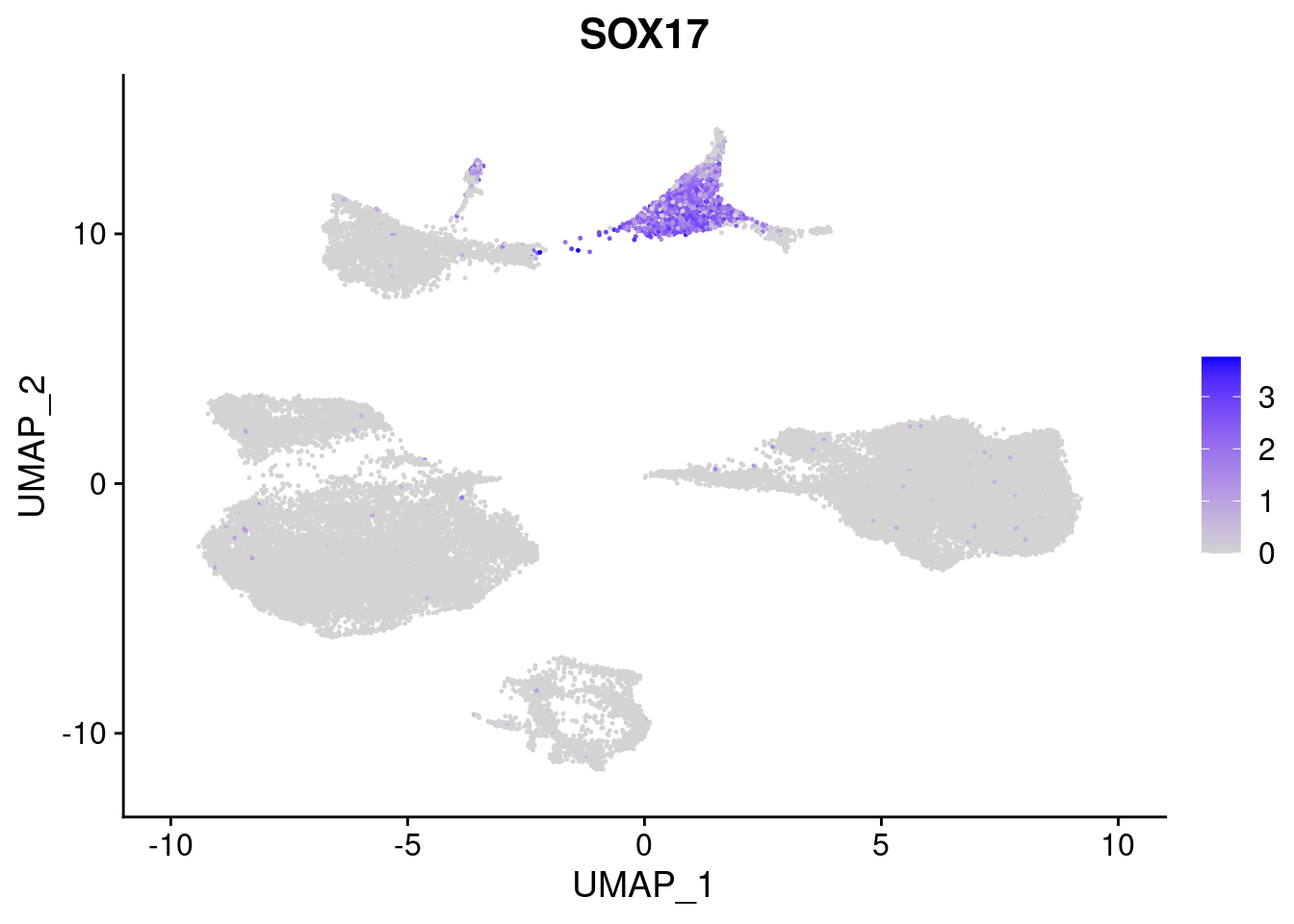
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[2]]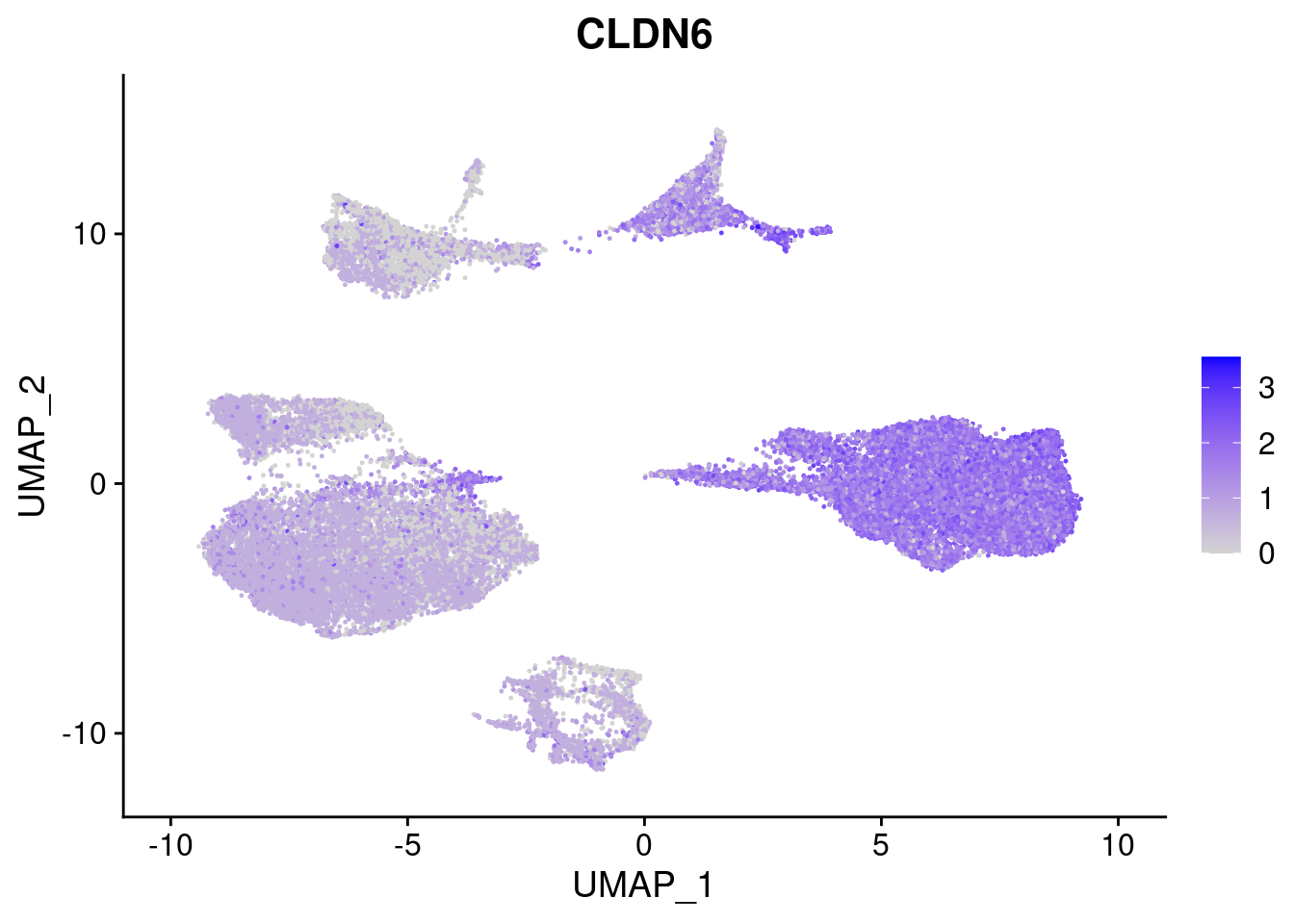
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[3]]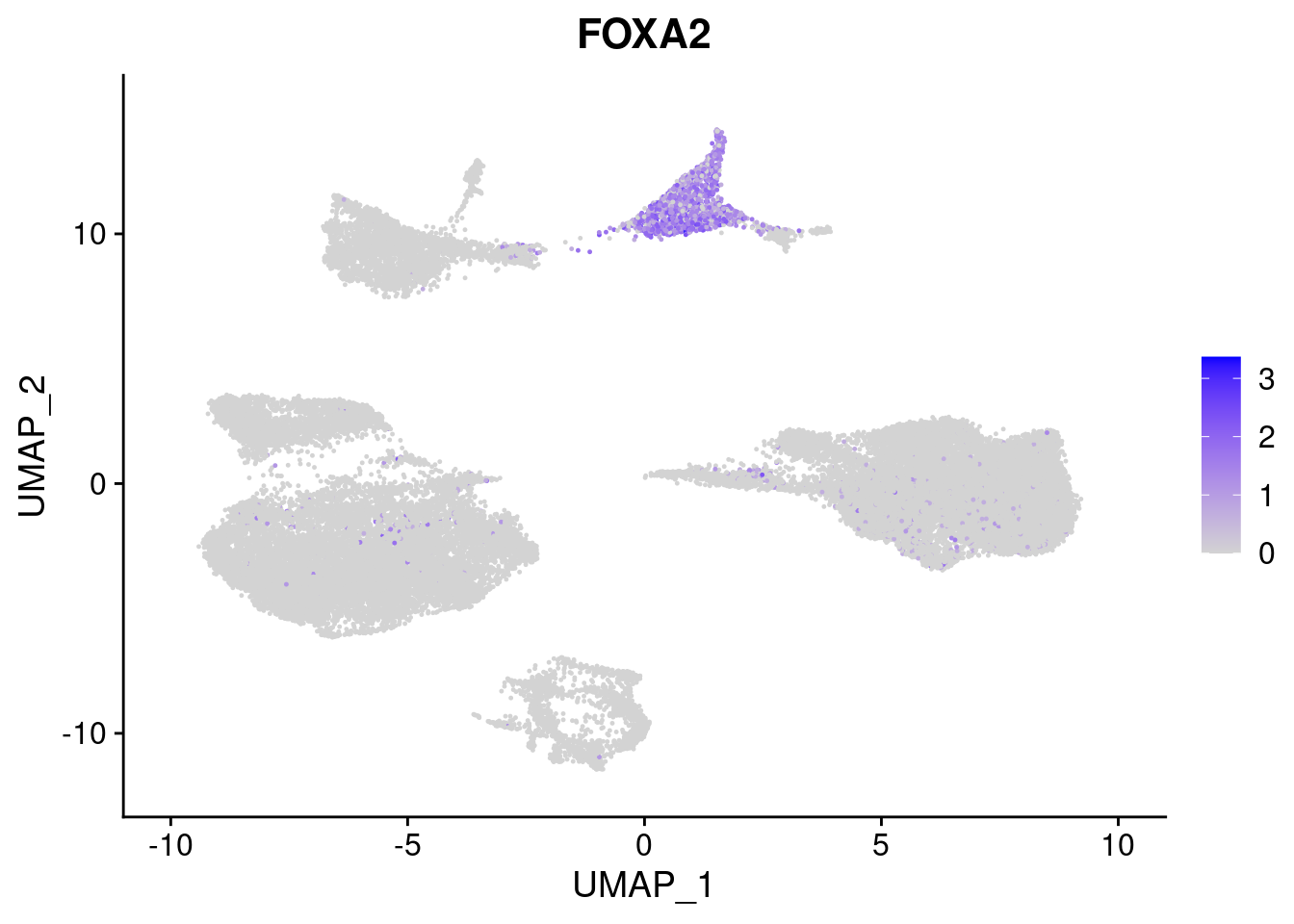
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[4]]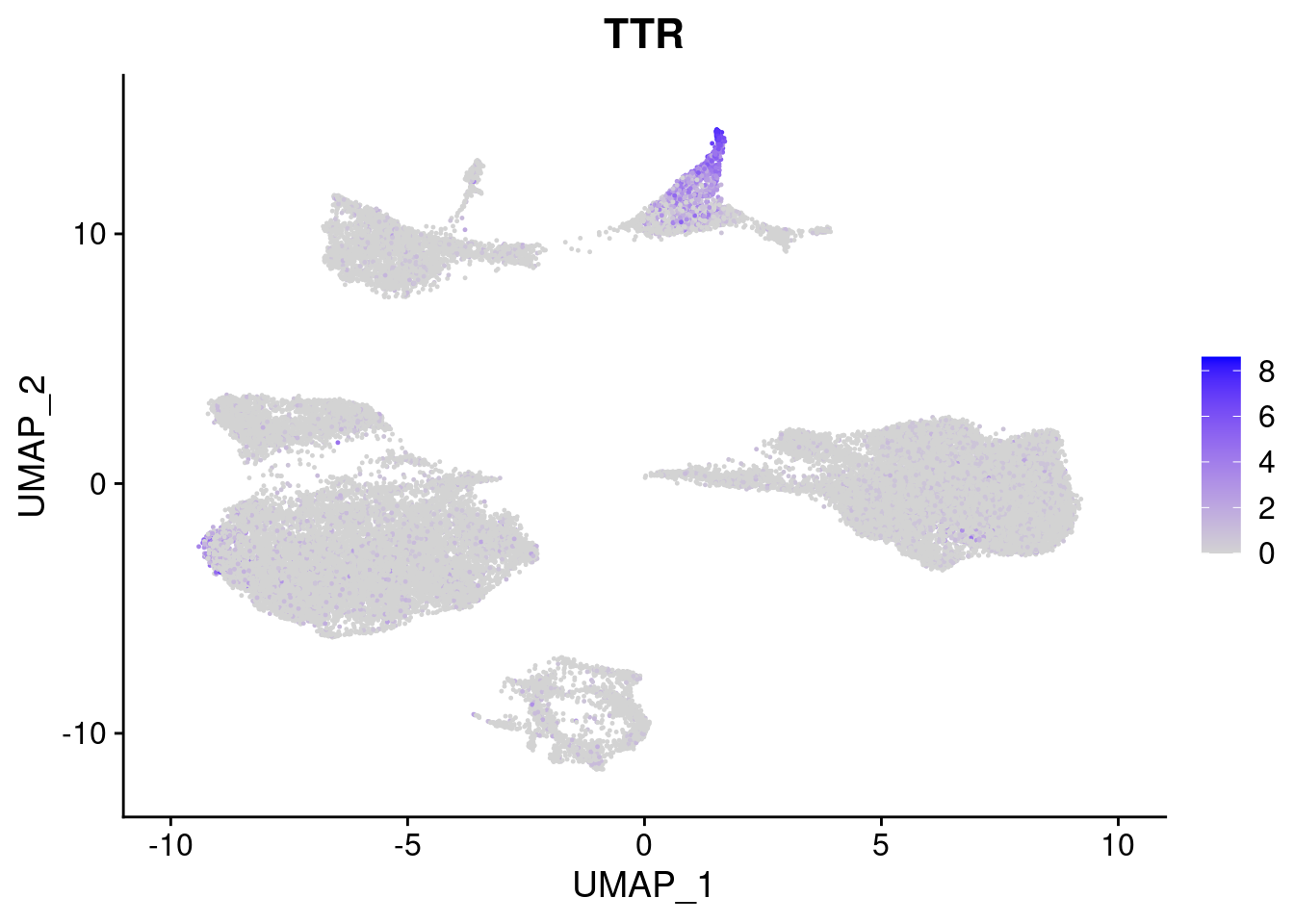
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[5]]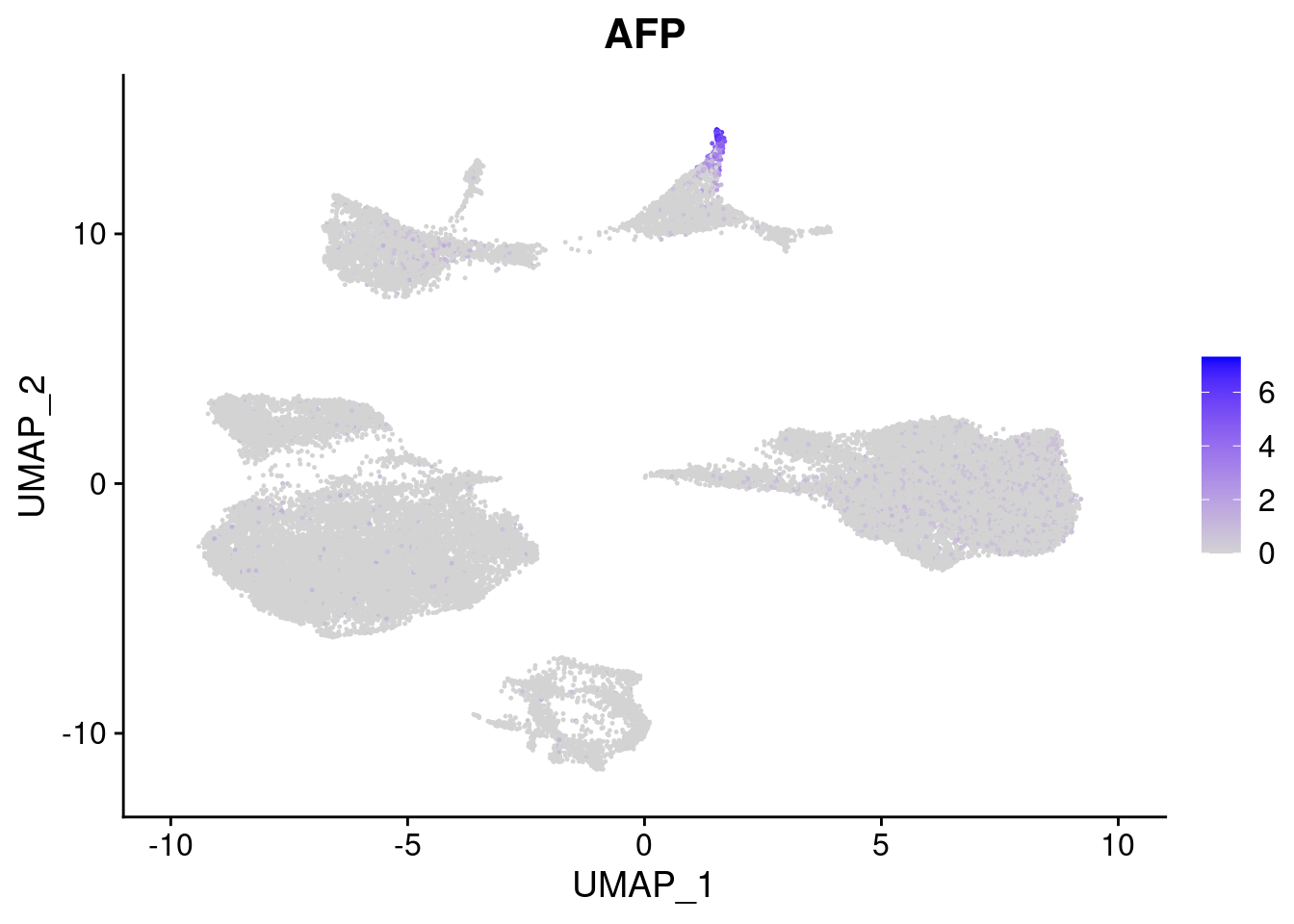
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[6]]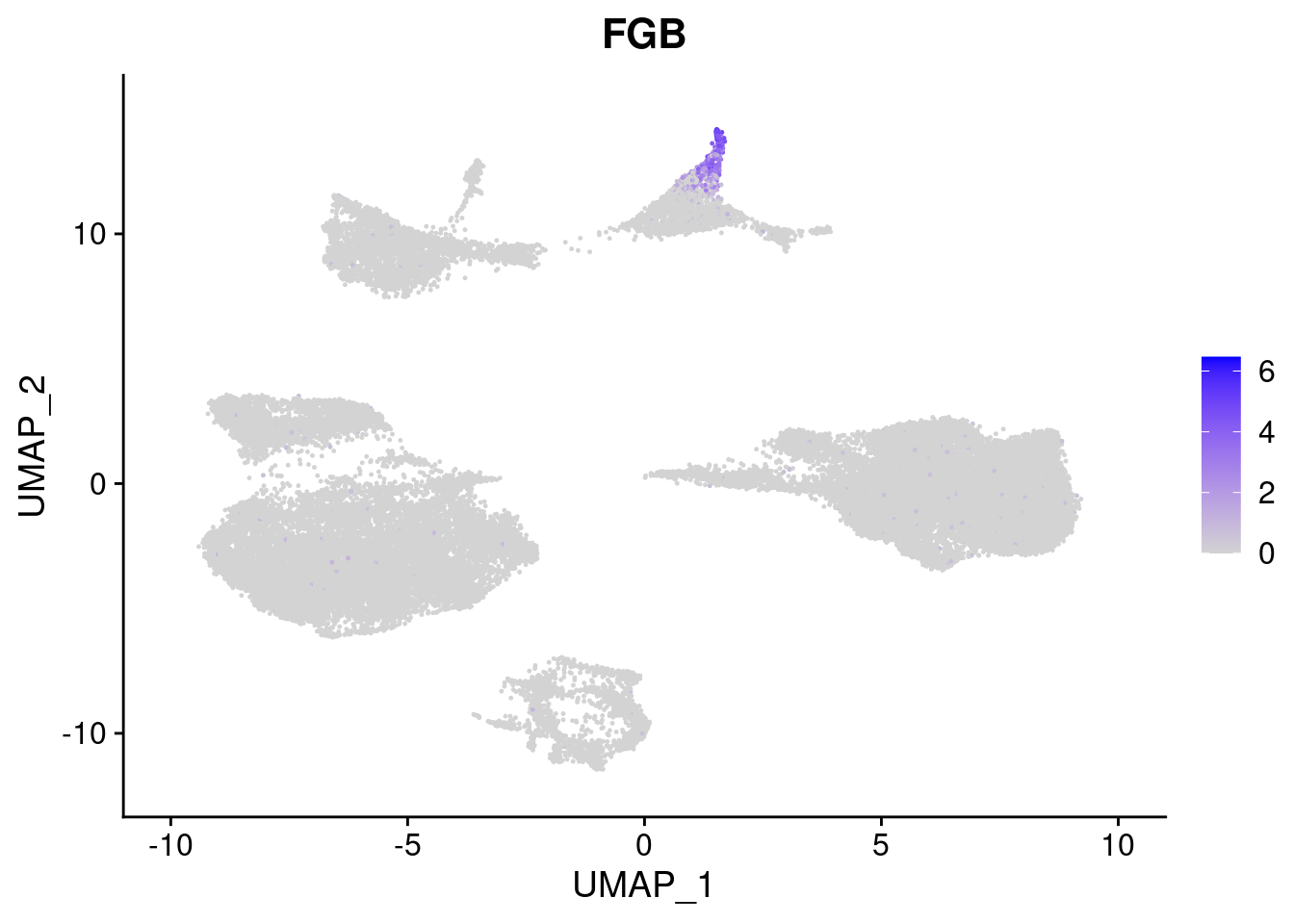
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#Mesoderm Markers (first 3 early meso markers, 4-6 heart markers, 7-9 endothelial markers (which comes from mesoderm), then some other general muscle markers)
FeaturePlot(merged, features = c("HAND1", "BMP4", "TNNT2","KDR", "GNG11", "ECSCR", "COL3A1", "ACTC1"), pt.size = 0.2, combine=F)[[1]]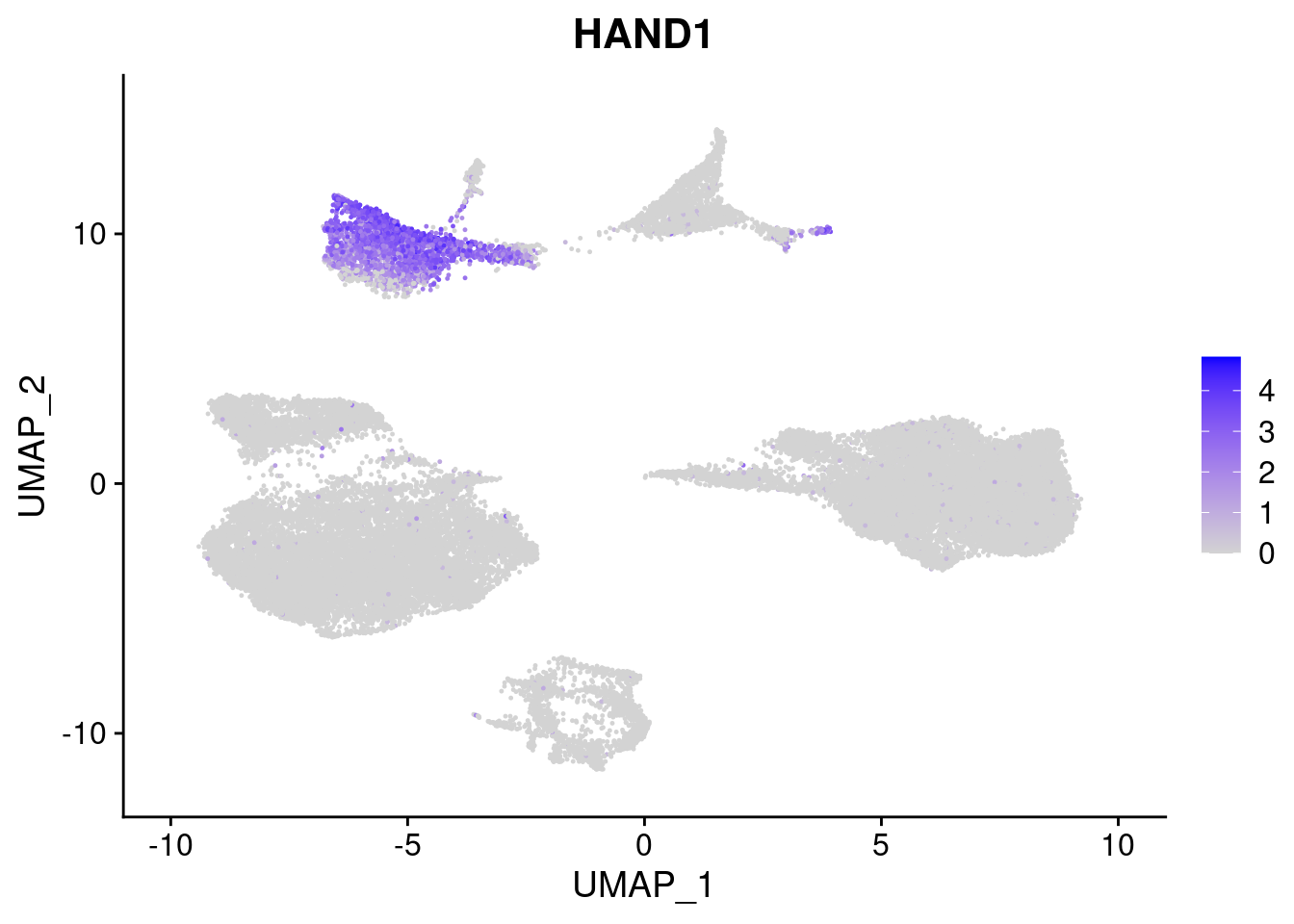
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[2]]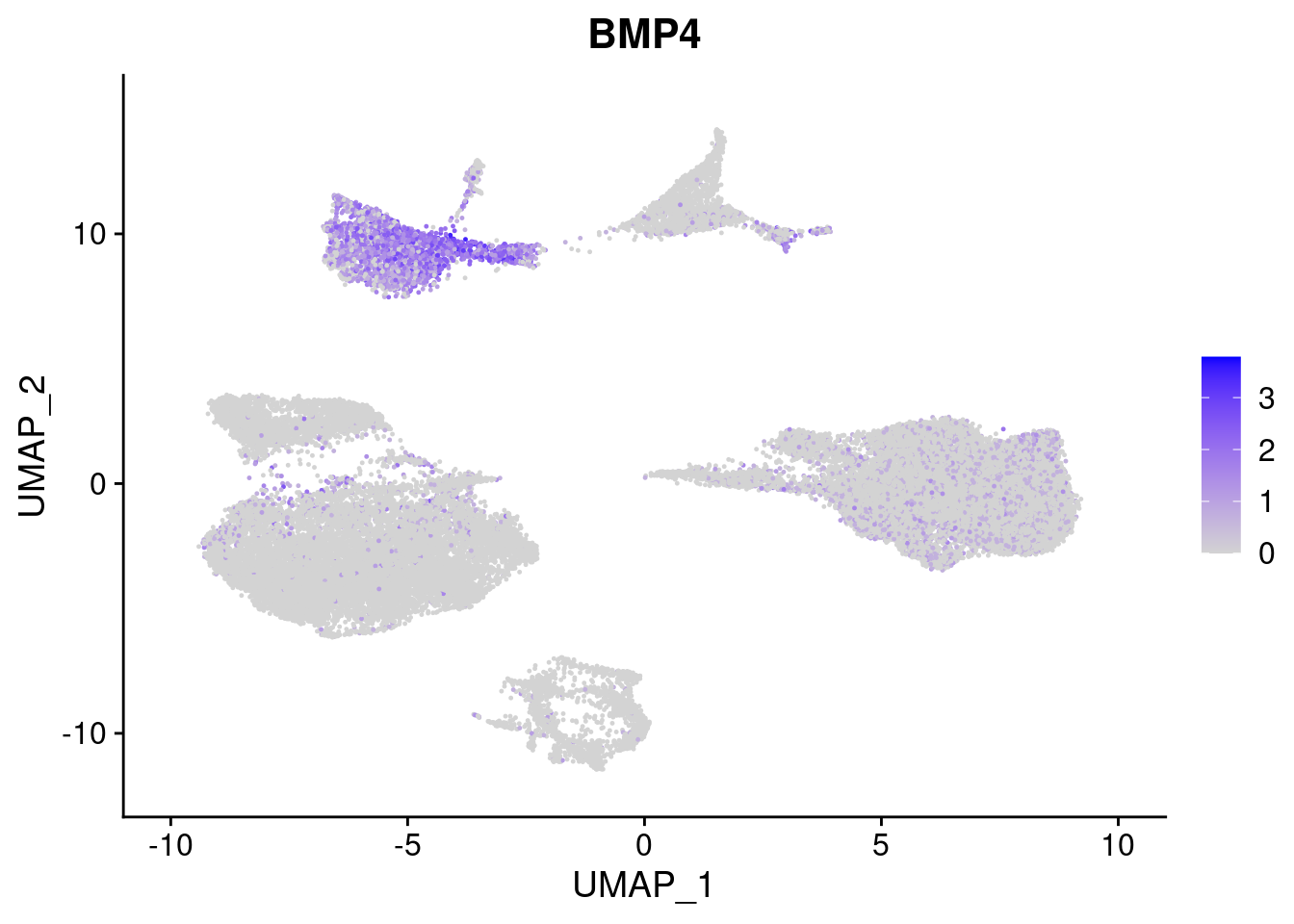
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[3]]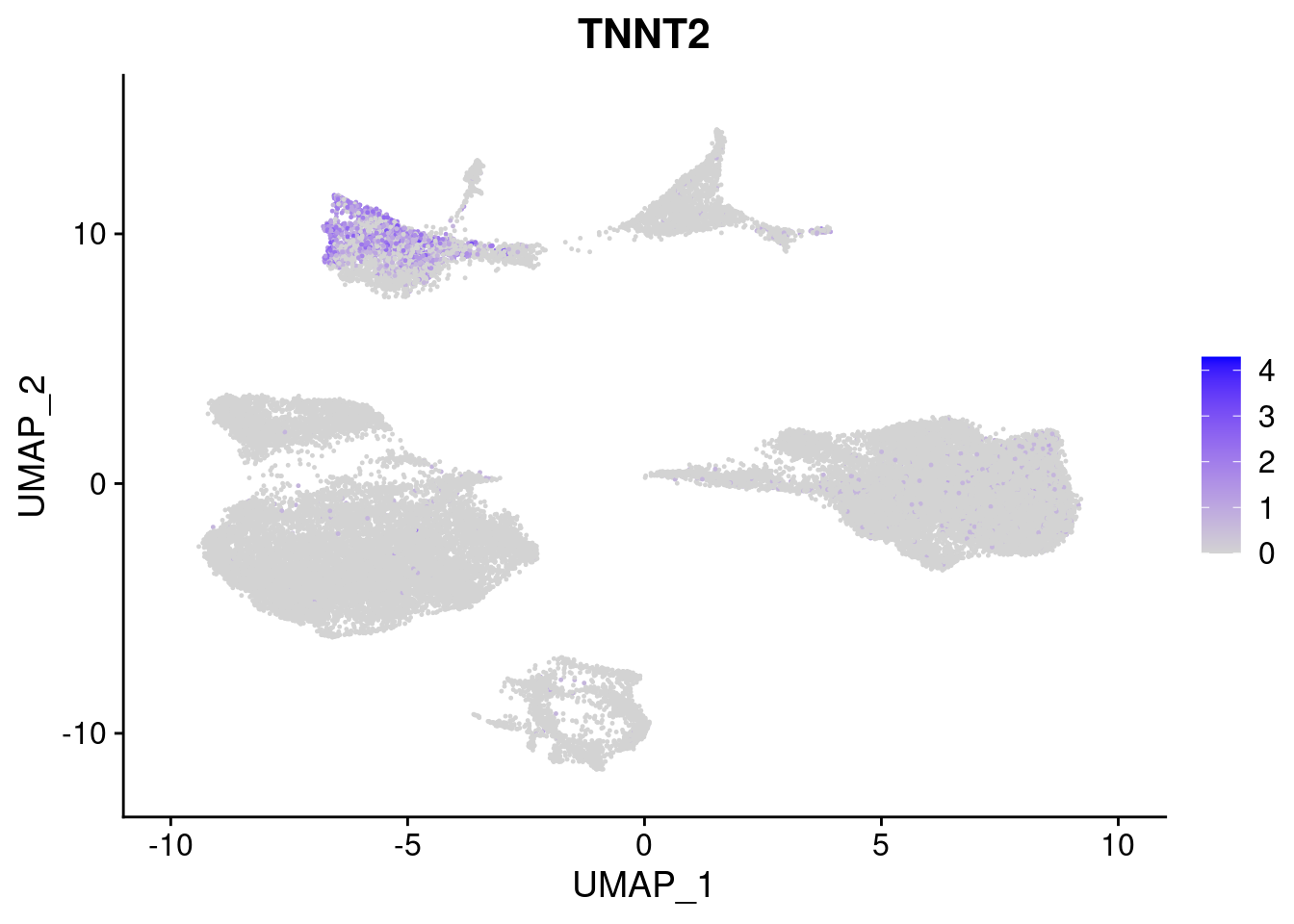
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[4]]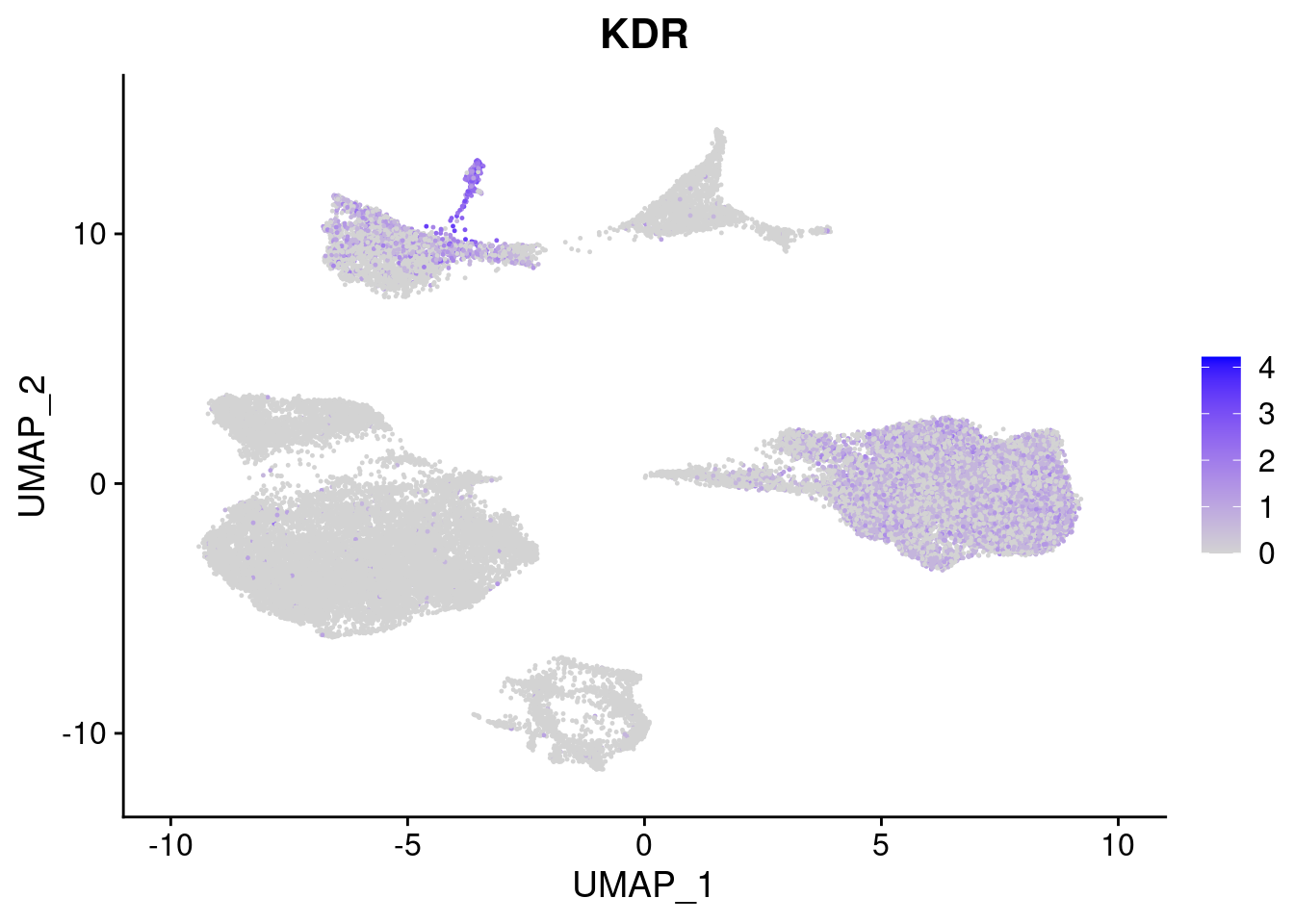
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[5]]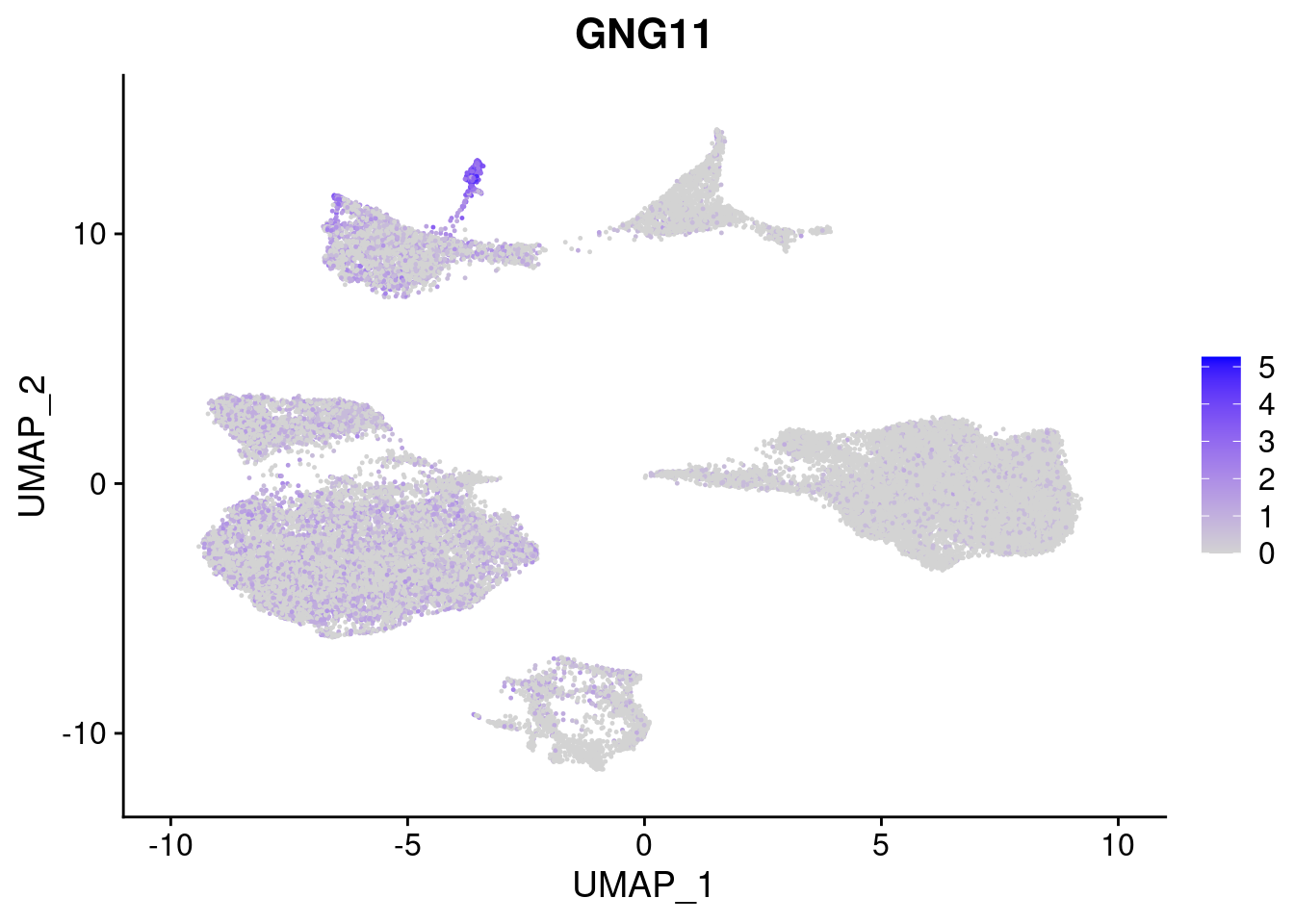
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[6]]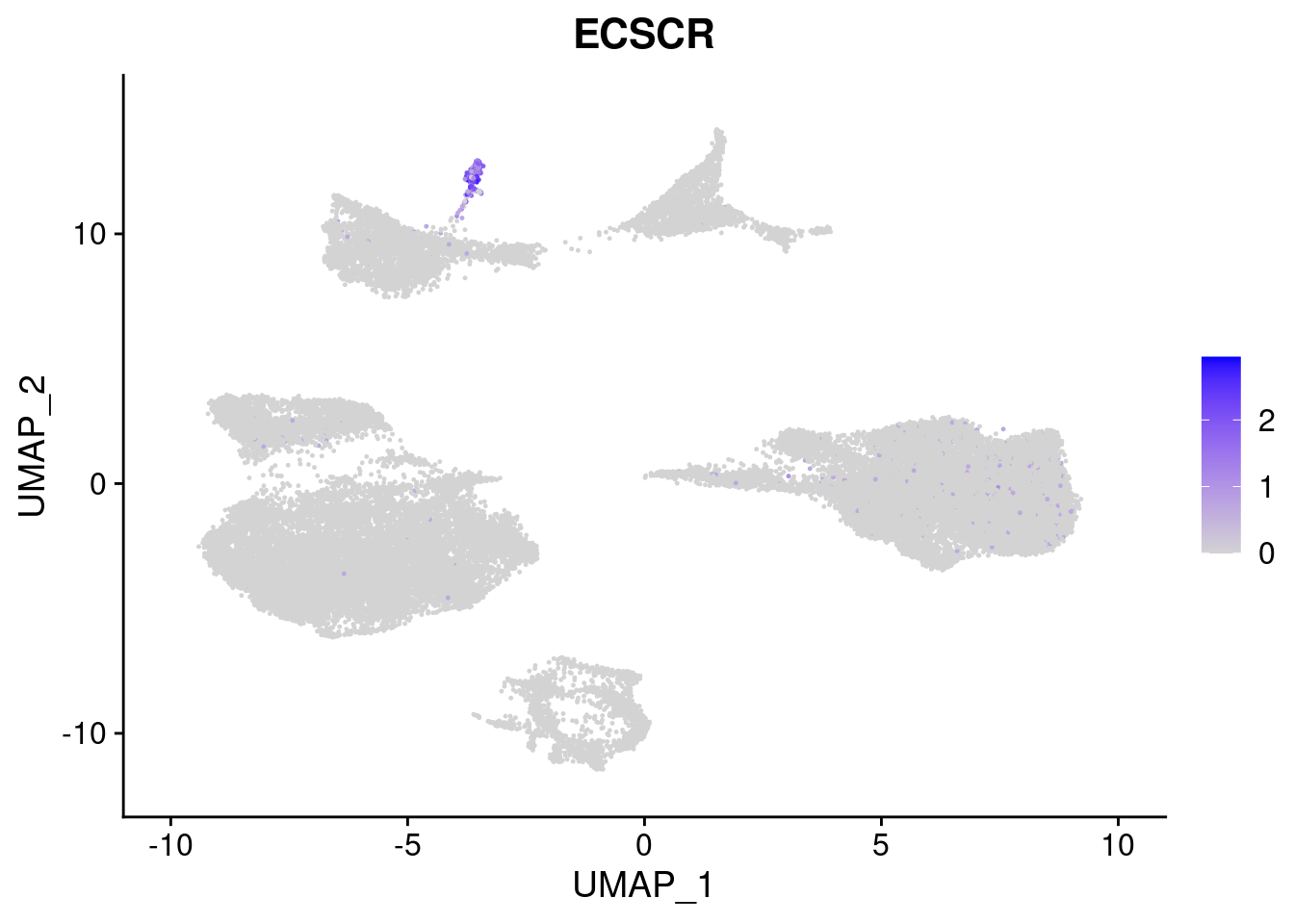
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[7]]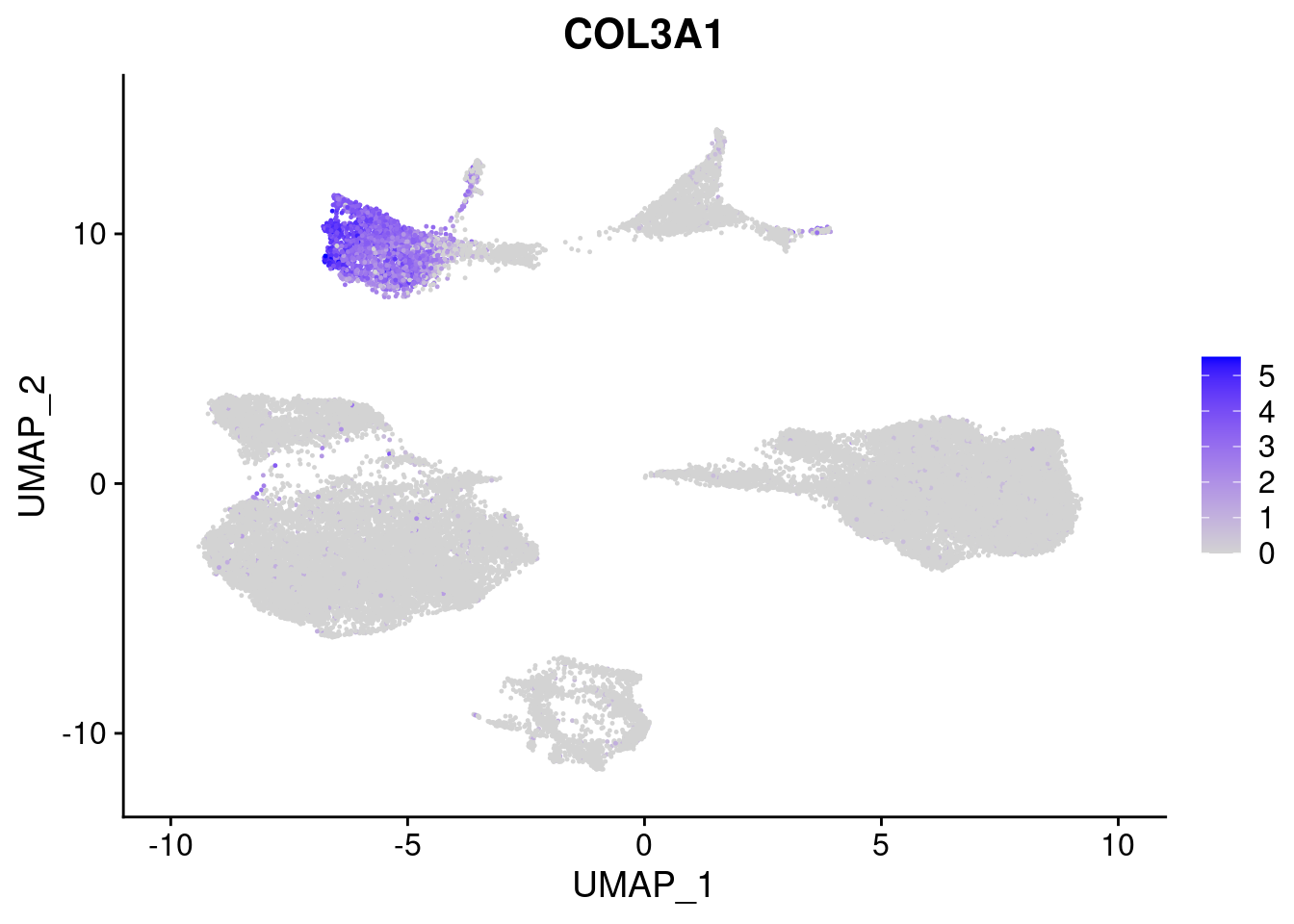
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[8]]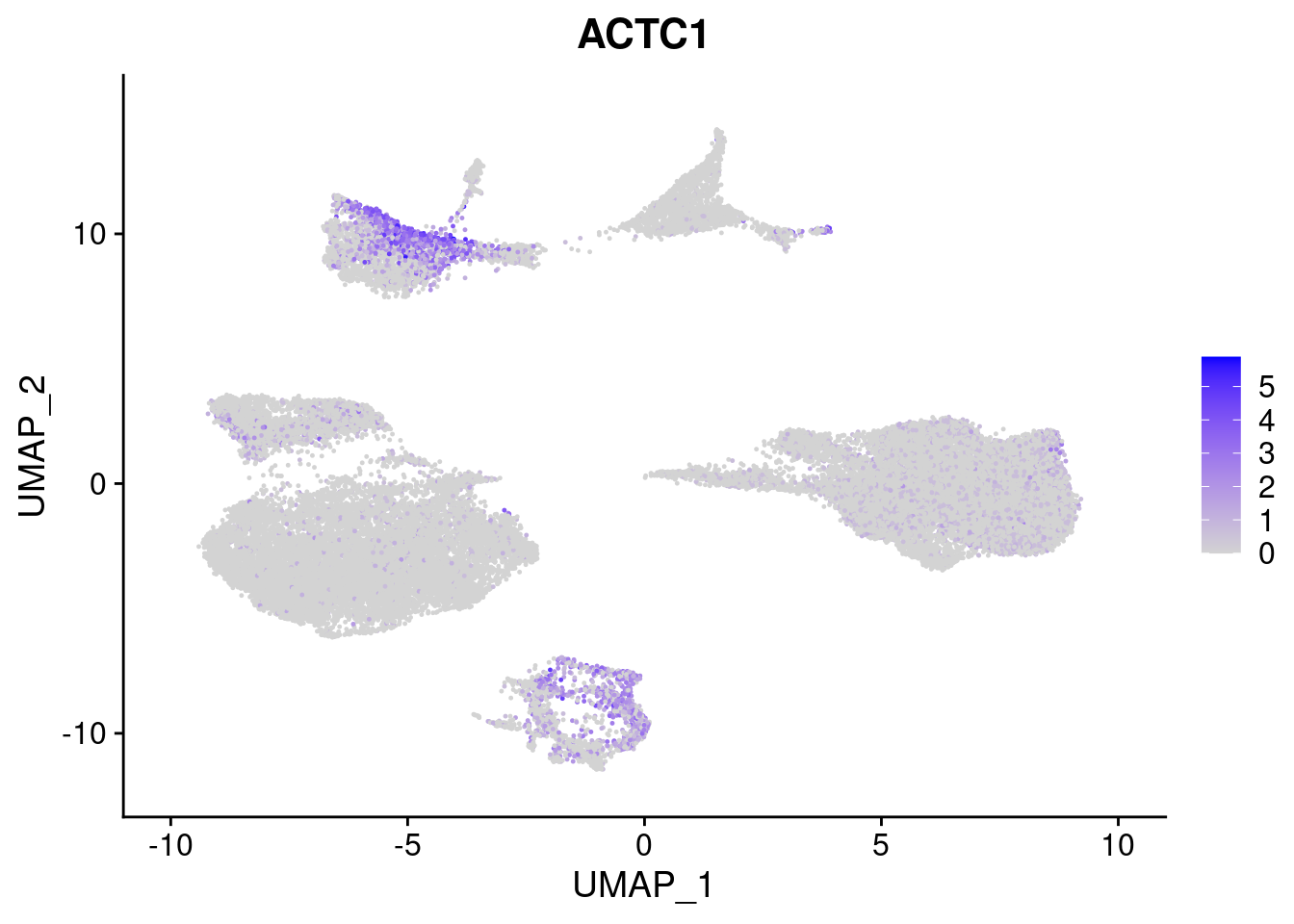
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#Ectoderm Markers (3-1 early ectoderm markers, 4-6schwann cell (myelinating, non myelinating, or precursor), 7-8 oligodendrocytes, 9-10 radial glia)
FeaturePlot(merged, features = c("PAX6", "GBX2", "NES", "MPZ", "SOX10","GAP43", "OLIG1", "OLIG2", "VIM", "HES5"), pt.size = 0.2, ncol=3, combine=F)[[1]]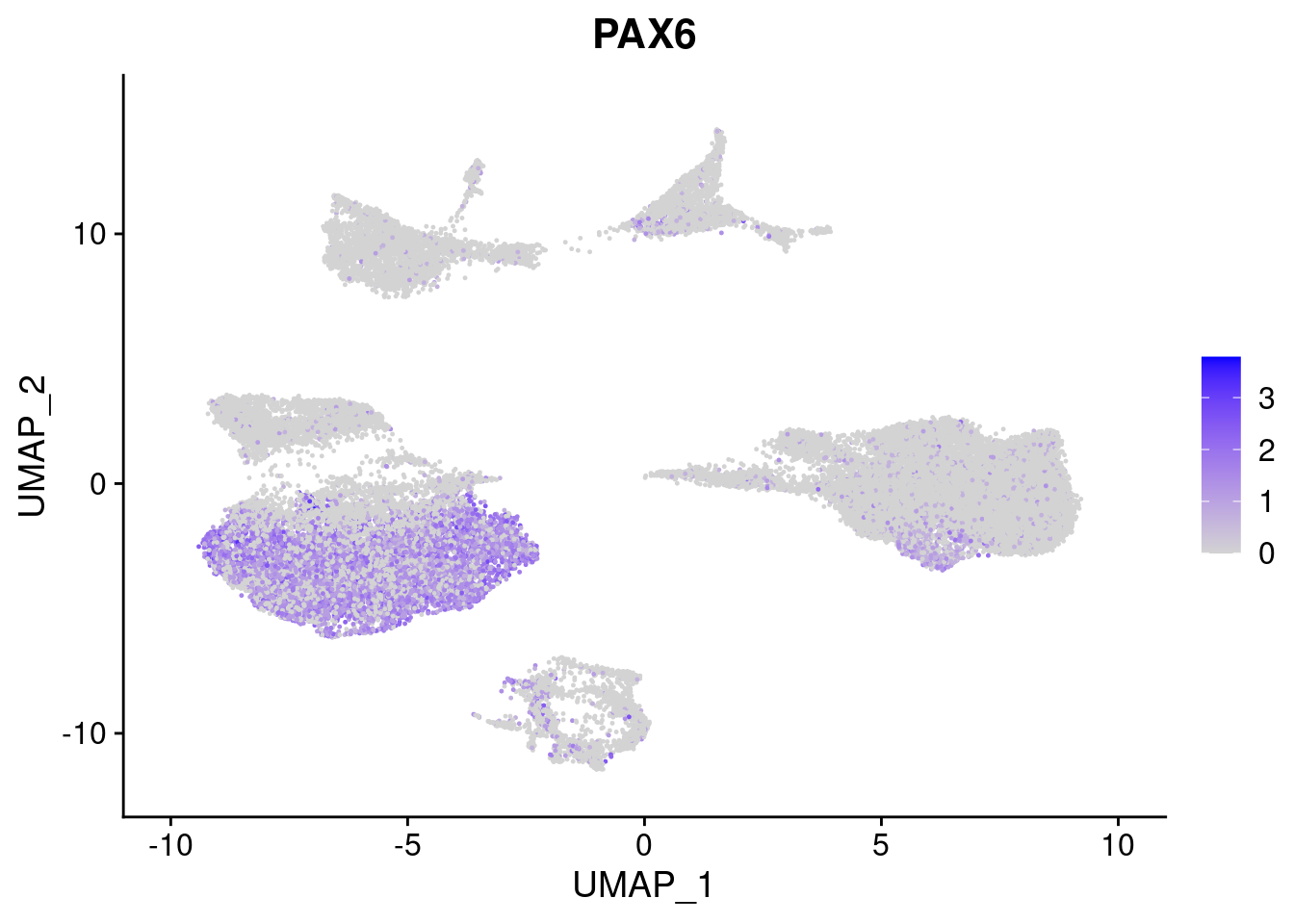
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[2]]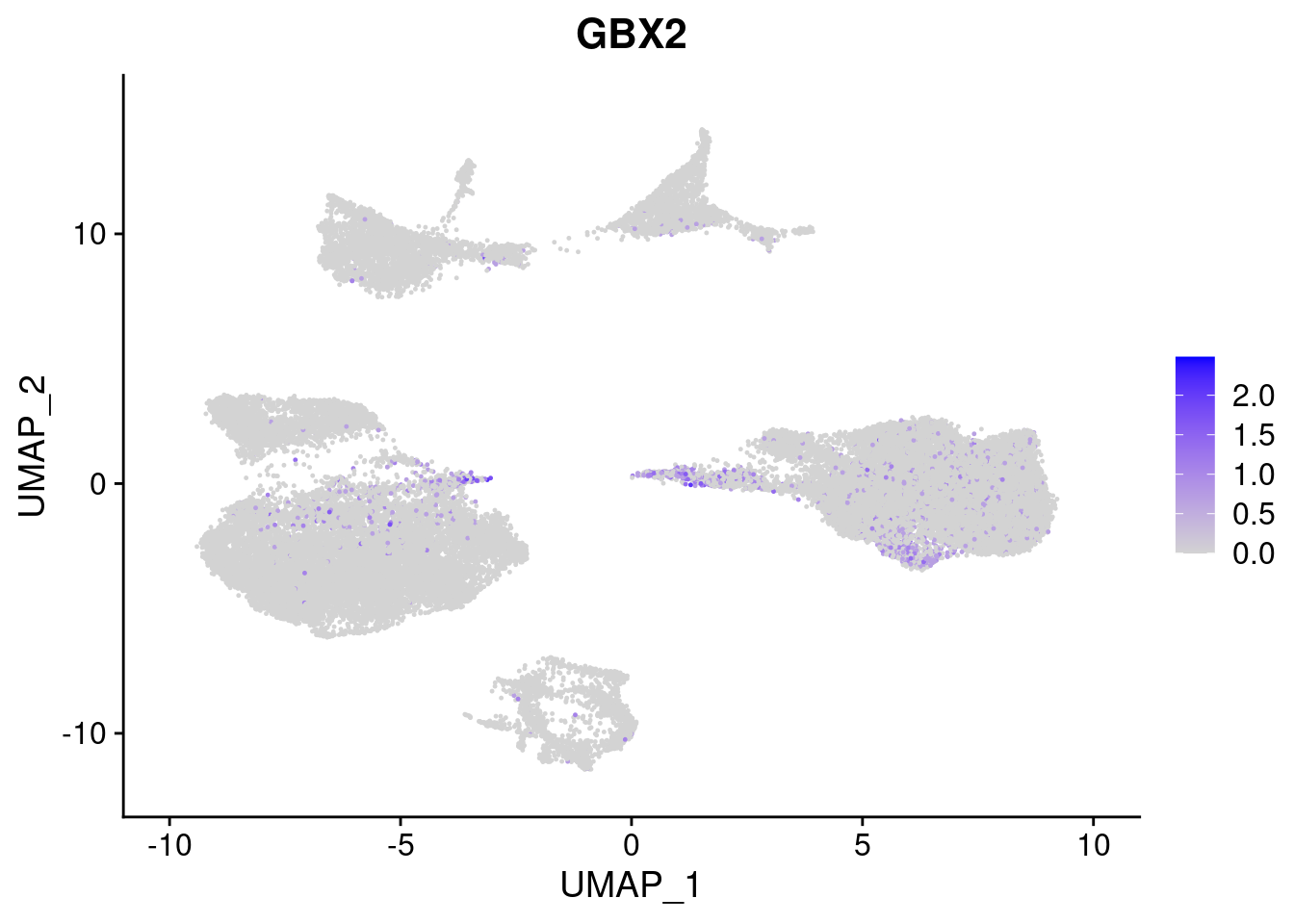
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[3]]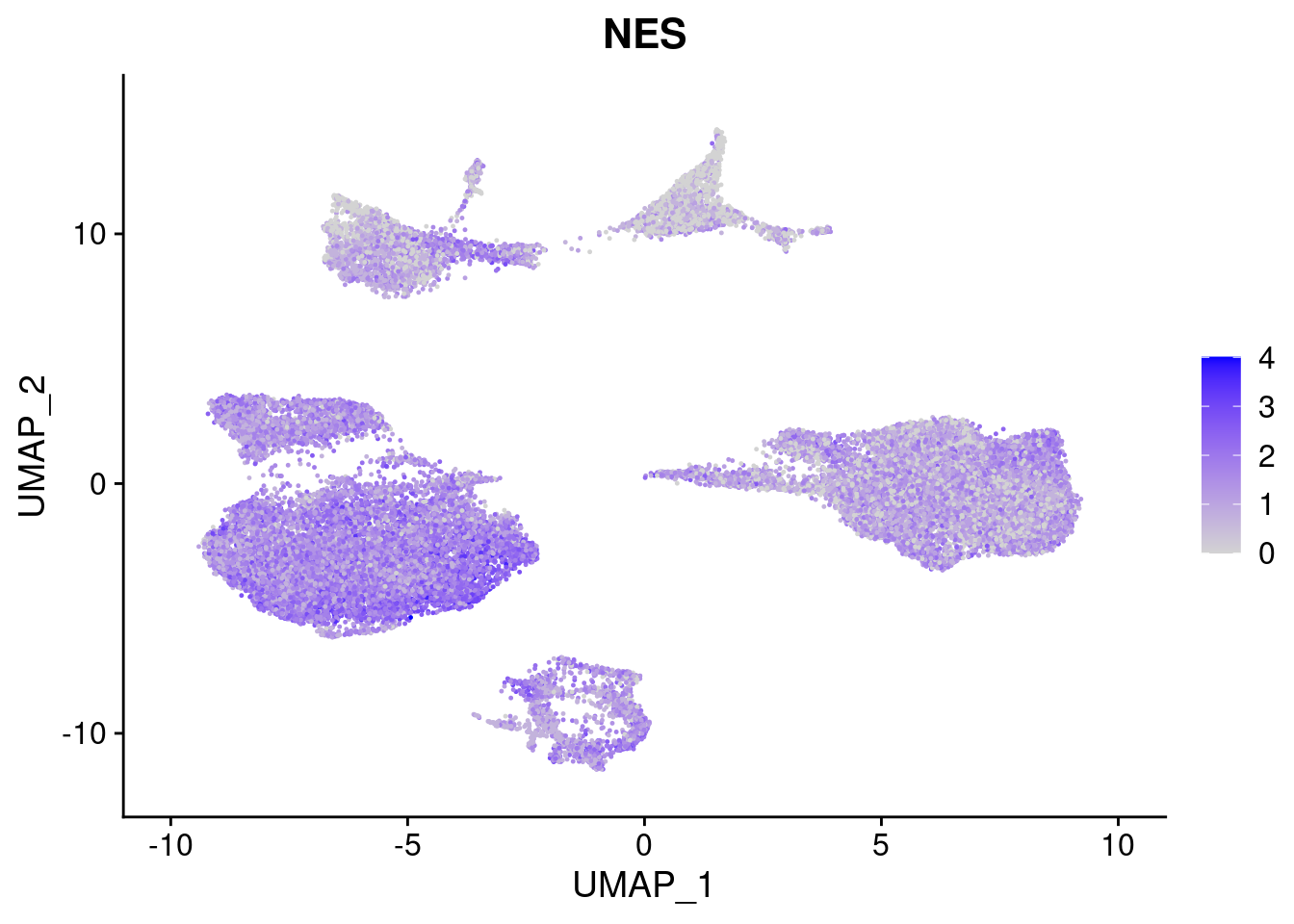
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[4]]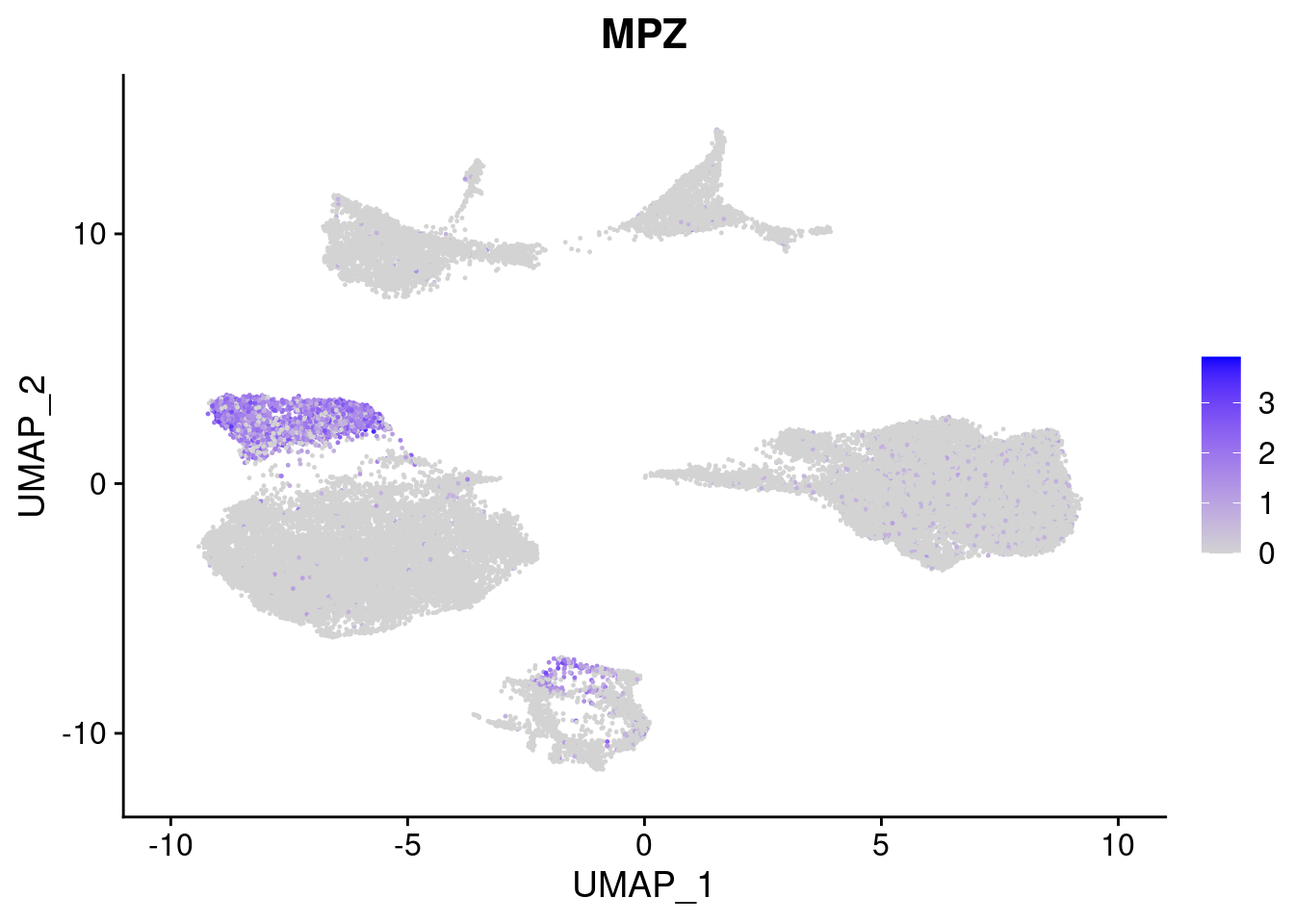
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[5]]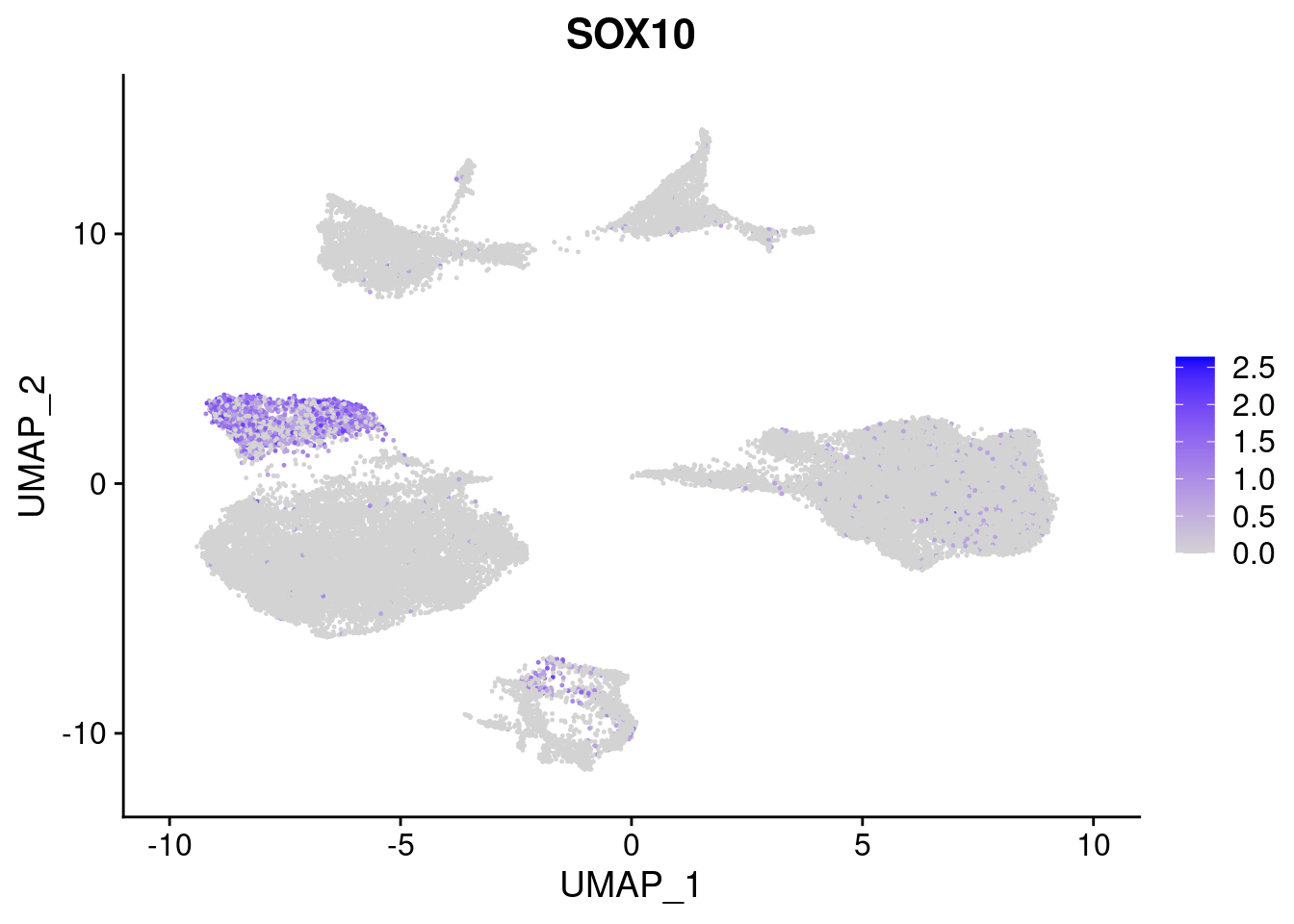
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[6]]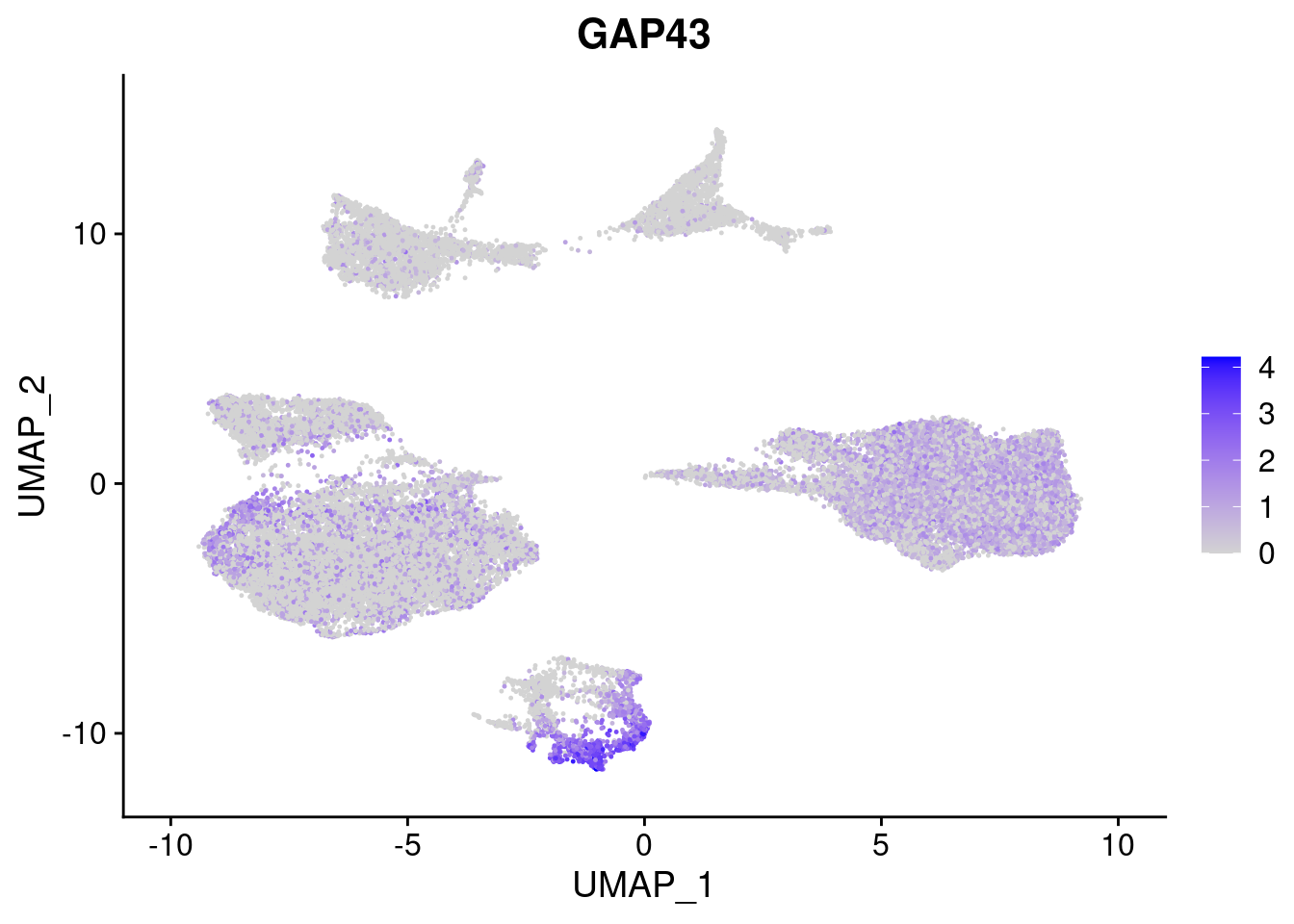
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[7]]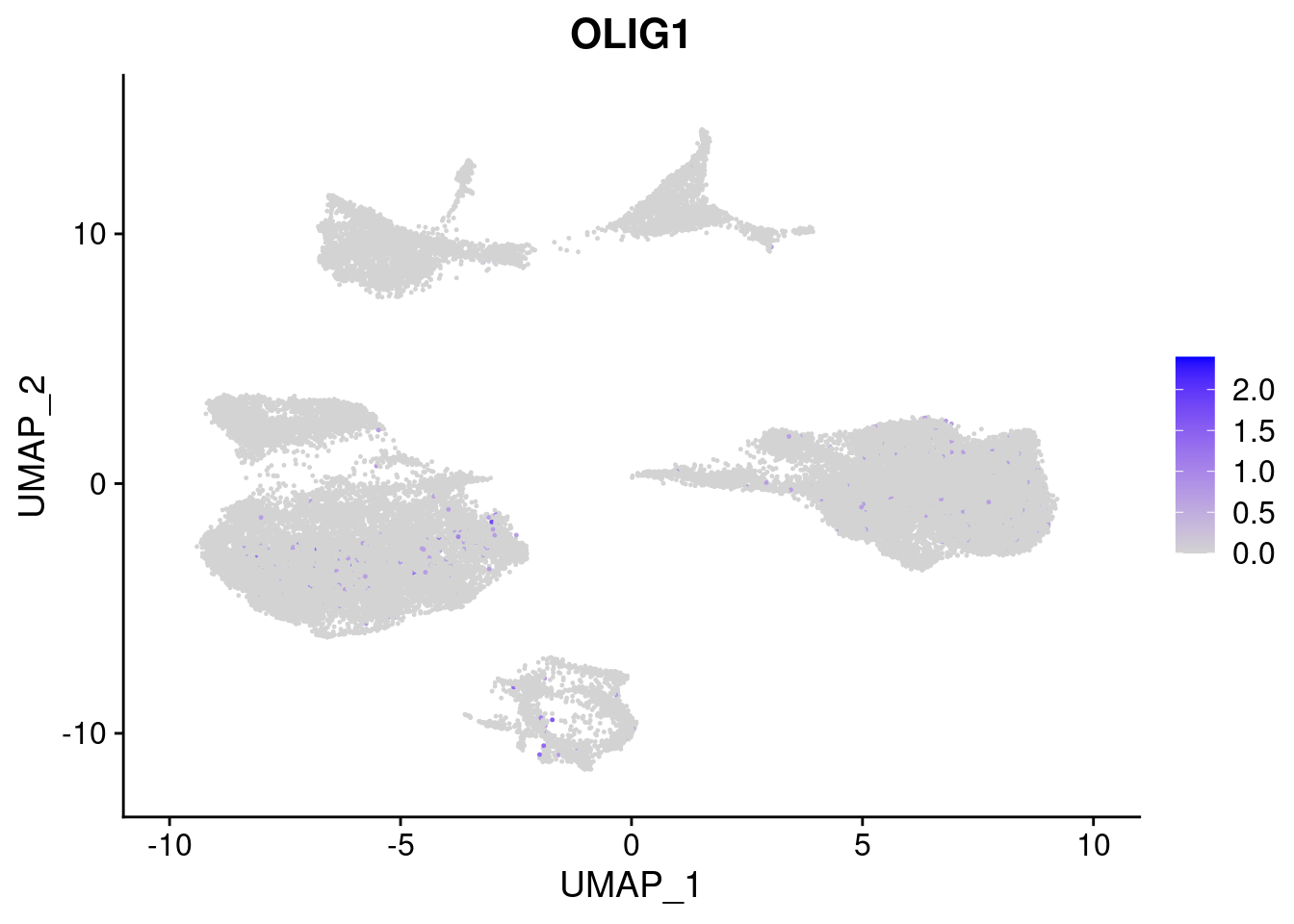
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[8]]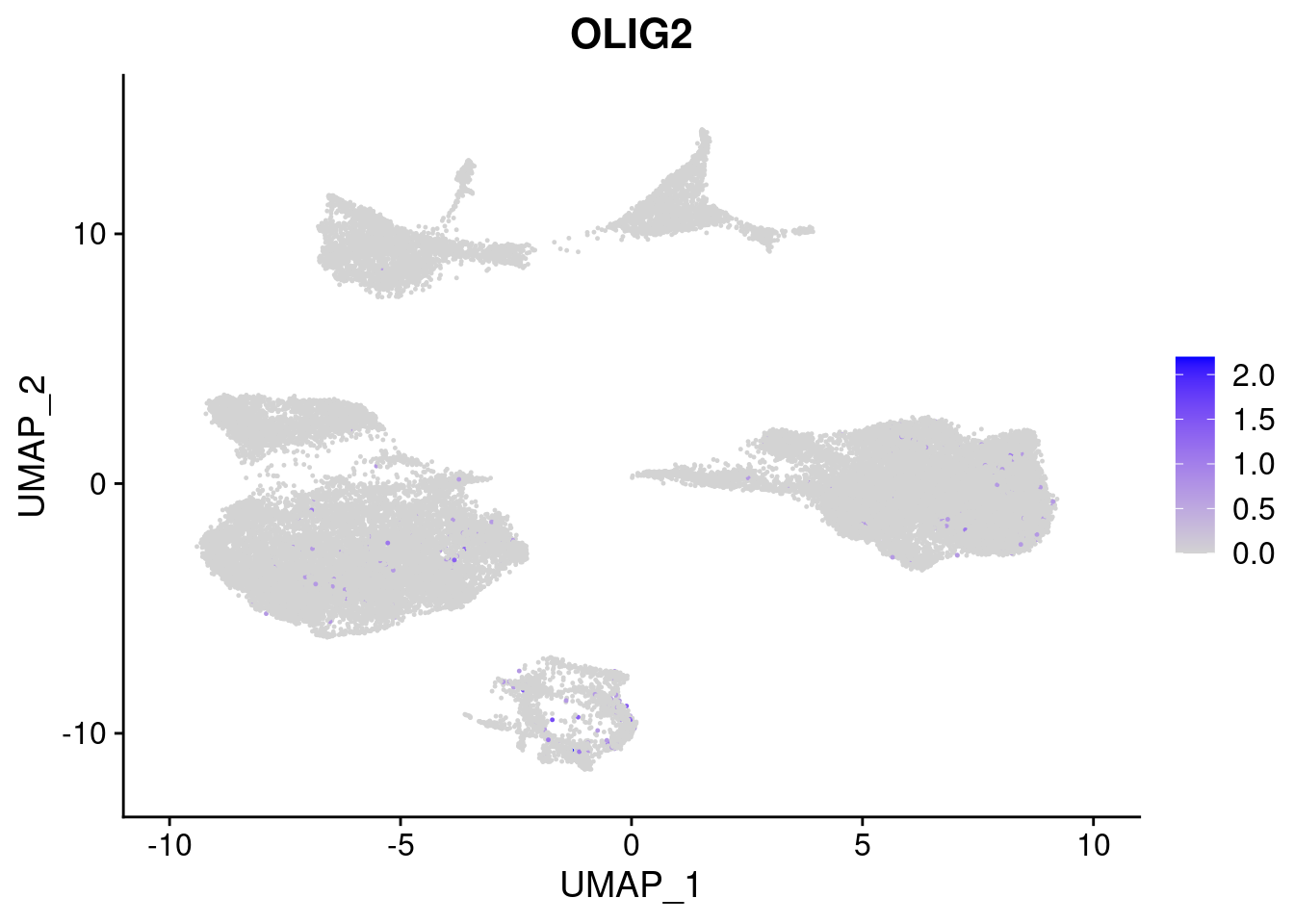
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[9]]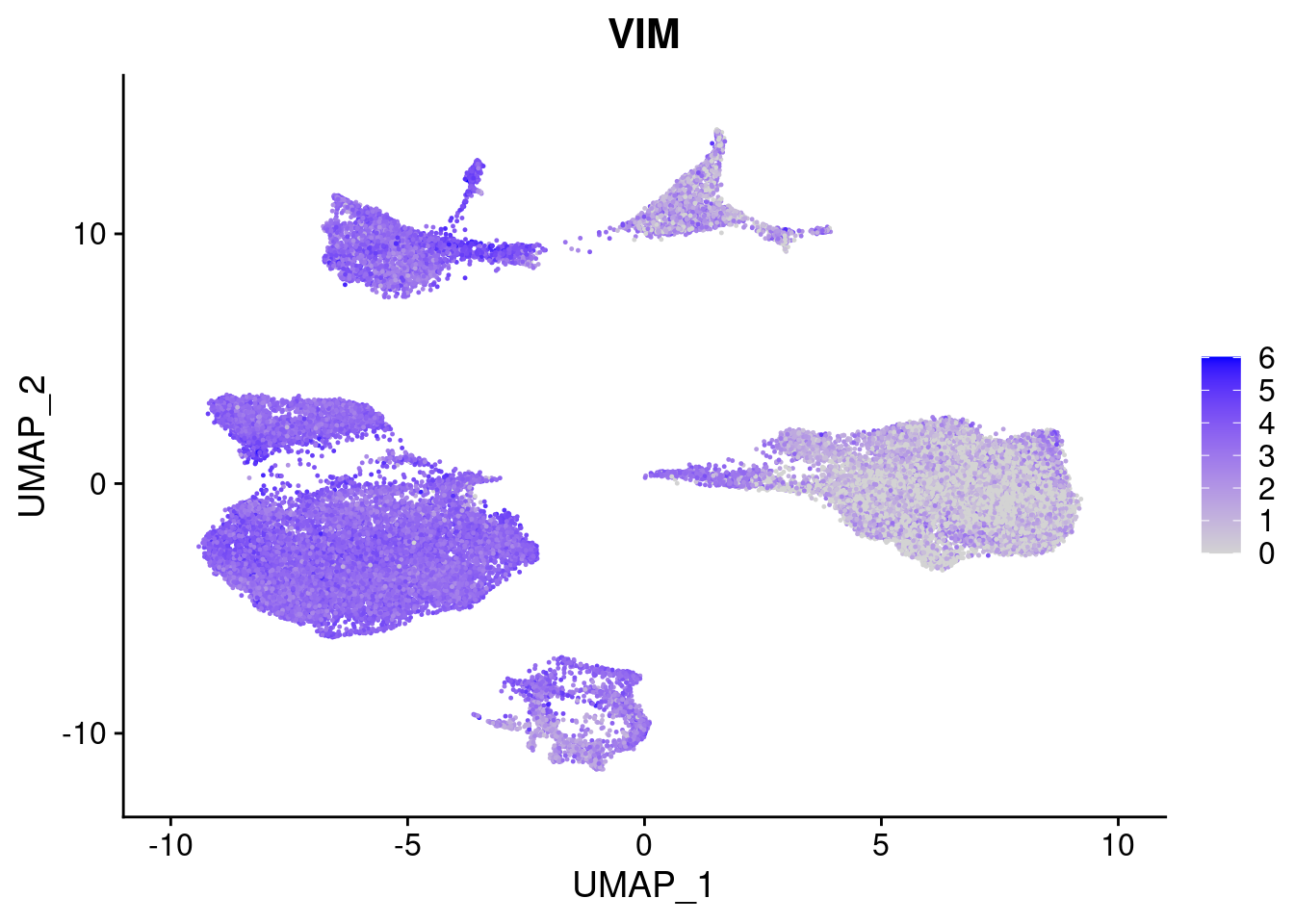
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
[[10]]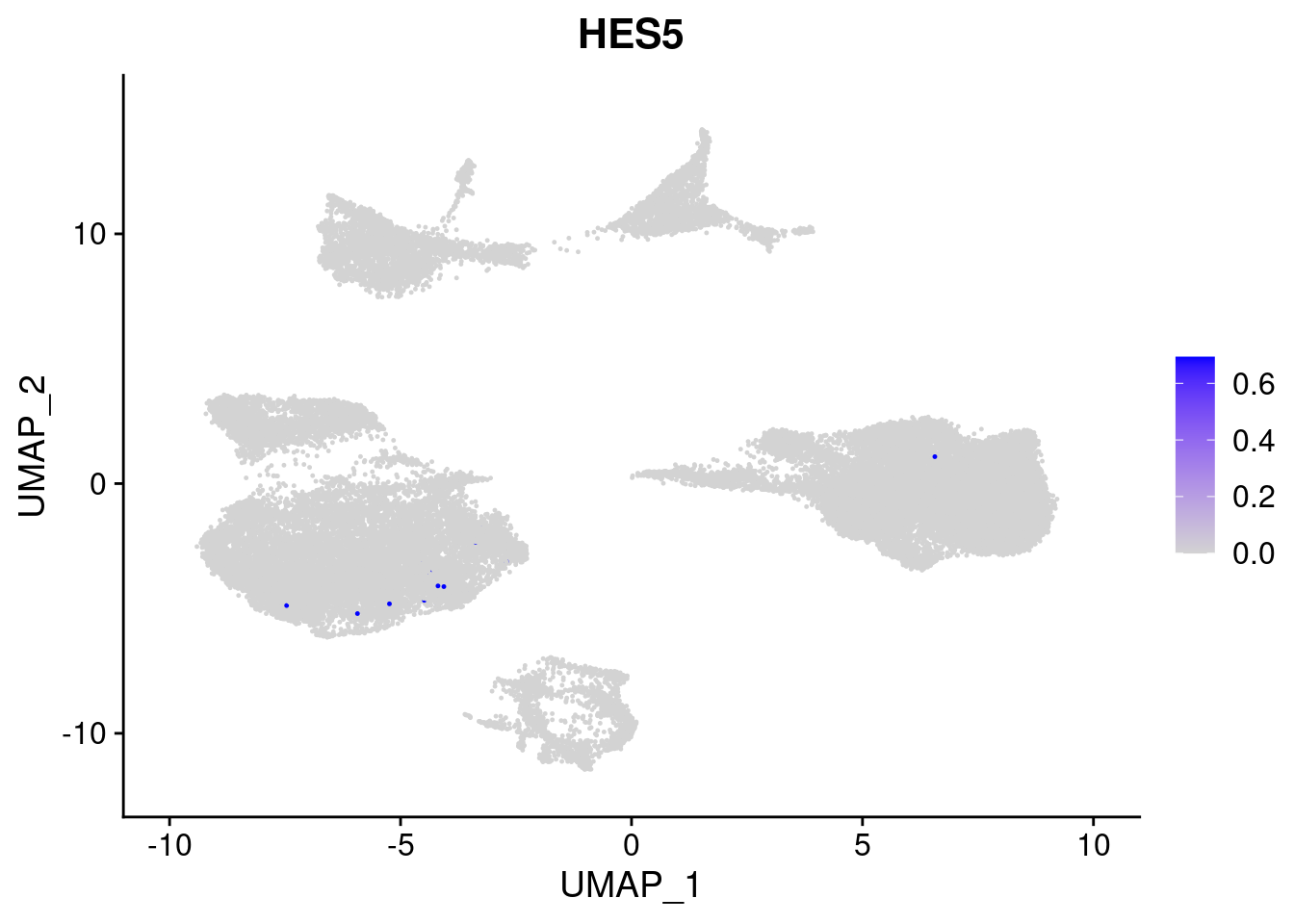
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#More ectoderm, specifically neurons
#immature neurons: NEUROD1
#Mature Neurons: MAP2, SYP
#dopaminergic: TH, FOXA2,
FeaturePlot(merged, features = c("MAP2", "SYP","NEUROD1", "TH" ), pt.size = 0.2, ncol=3)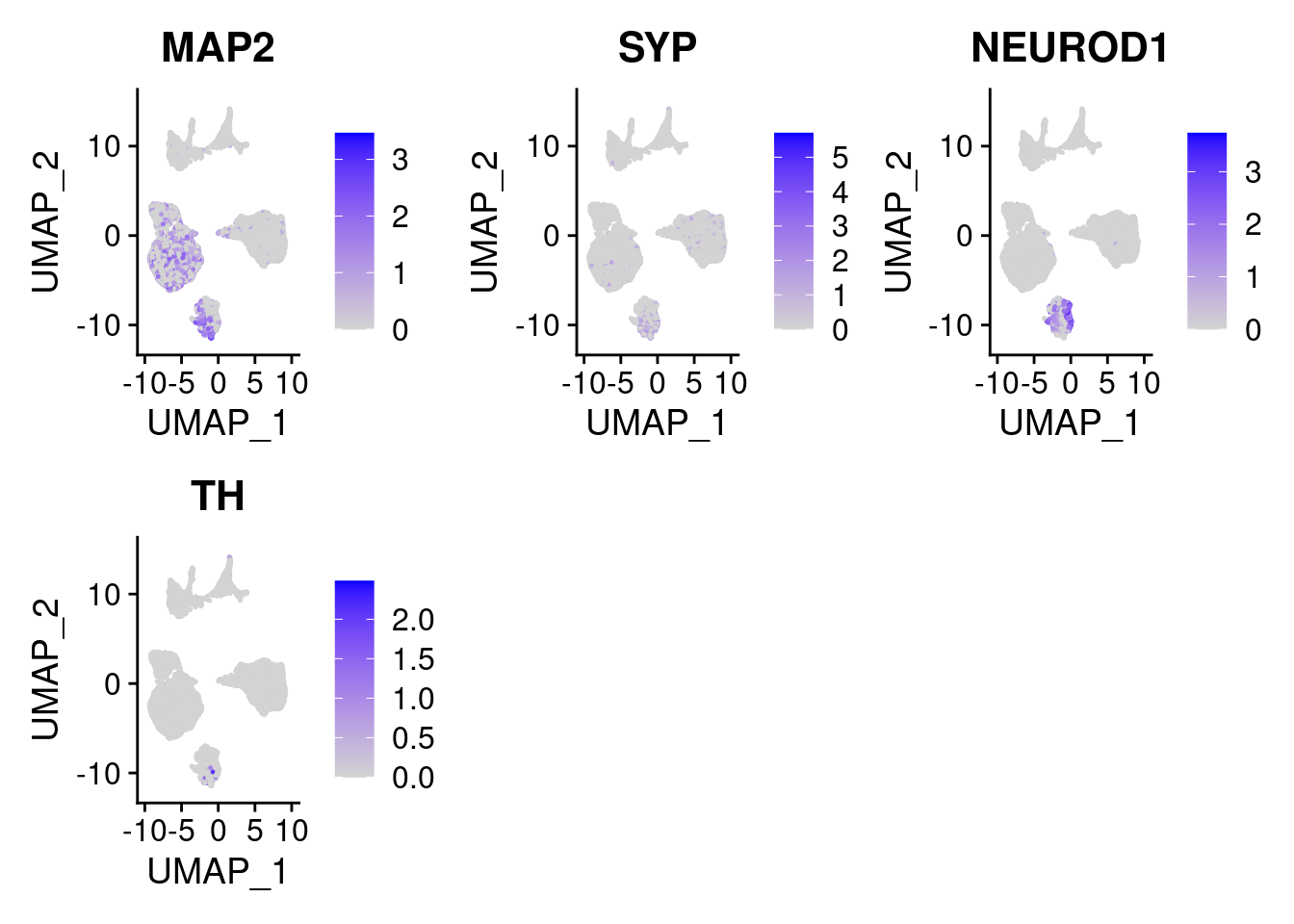
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
Identify cluster markers
#how many cells per cluster?
t1<-table(merged@meta.data$SCT_snn_res.1, merged@meta.data$Batch)
t1
Batch1 Batch2 Batch3
0 3608 1884 2989
1 2213 1263 1191
2 1964 872 907
3 1243 849 1510
4 1359 427 870
5 443 854 776
6 794 239 549
7 560 469 421
8 255 276 835
9 353 288 681
10 457 427 360
11 436 178 386
12 357 116 427
13 186 266 428
14 471 156 247
15 344 193 301
16 13 56 693
17 273 242 219
18 32 83 551
19 115 125 376
20 270 117 220
21 294 140 36
22 82 77 245
23 218 60 106
24 135 71 98
25 102 60 135
26 92 37 100
27 118 23 33
28 86 17 60#percent of cells in each cluster per batch
t1colsum<- colSums(t1)
percT1<-t1/t1colsum
percT1
Batch1 Batch2 Batch3
0 0.213832751 0.119619048 0.302990370
1 0.224328434 0.074853316 0.075619048
2 0.124698413 0.088393310 0.053754519
3 0.073667990 0.053904762 0.153066396
4 0.137759757 0.025306703 0.055238095
5 0.028126984 0.086568677 0.045990636
6 0.047057429 0.015174603 0.055651292
7 0.056766346 0.027795887 0.026730159
8 0.016190476 0.027977699 0.049487347
9 0.020920998 0.018285714 0.069031931
10 0.046325393 0.025306703 0.022857143
11 0.027682540 0.018043588 0.022876785
12 0.021158063 0.007365079 0.043284339
13 0.018854536 0.015764831 0.027174603
14 0.029904762 0.015813482 0.014638772
15 0.020387601 0.012253968 0.030511911
16 0.001317790 0.003318912 0.044000000
17 0.017333333 0.024531171 0.012979316
18 0.001896521 0.005269841 0.055854029
19 0.011657375 0.007408285 0.023873016
20 0.017142857 0.011860112 0.013038582
21 0.017424287 0.008888889 0.003649265
22 0.008312215 0.004563504 0.015555556
23 0.013841270 0.006082108 0.006282226
24 0.008000948 0.004507937 0.009934110
25 0.010339584 0.003555977 0.008571429
26 0.005841270 0.003750634 0.005926628
27 0.006993421 0.001460317 0.003345160
28 0.008717689 0.001007527 0.003809524heatmap(t(percT1))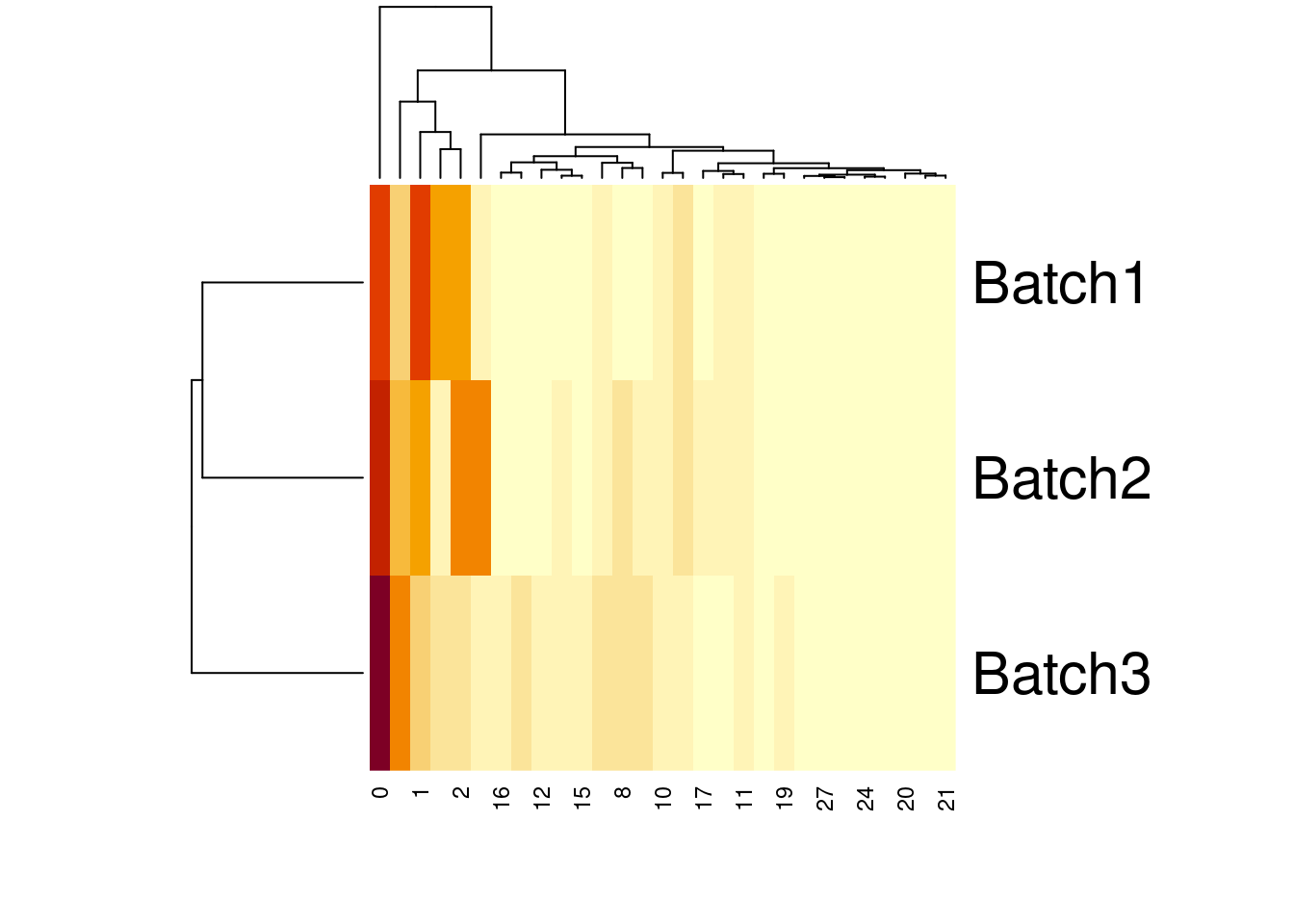
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#how many cells per cluster from each individual?
t2<-table(merged@meta.data$SCT_snn_res.1, merged@meta.data$individual)
t2
SNG-NA18511 SNG-NA18858 SNG-NA19160
0 322 7991 168
1 3094 237 1336
2 1758 271 1714
3 223 3294 85
4 824 38 1794
5 125 1803 145
6 167 41 1374
7 937 107 406
8 512 55 799
9 627 162 533
10 112 1026 106
11 121 13 866
12 84 12 804
13 349 140 391
14 88 18 768
15 543 39 256
16 487 6 269
17 416 61 257
18 399 3 264
19 163 20 433
20 55 528 24
21 137 310 23
22 111 6 287
23 86 105 193
24 101 4 199
25 89 6 202
26 18 201 10
27 1 173 0
28 0 0 163t2colsums<-colSums(t2)
percT2<- t2/t2colsums
percT2
SNG-NA18511 SNG-NA18858 SNG-NA19160
0 2.694786e-02 5.761771e-01 1.007798e-02
1 1.856029e-01 1.983430e-02 9.632994e-02
2 1.267575e-01 1.625675e-02 1.434430e-01
3 1.866265e-02 2.375081e-01 5.098980e-03
4 4.943011e-02 3.180182e-03 1.293532e-01
5 9.012906e-03 1.081584e-01 1.213491e-02
6 1.397606e-02 2.956233e-03 8.242352e-02
7 5.620876e-02 8.954724e-03 2.927392e-02
8 3.691686e-02 3.299340e-03 6.686752e-02
9 5.247301e-02 1.168073e-02 3.197361e-02
10 6.718656e-03 8.586493e-02 7.642945e-03
11 8.724493e-03 7.798440e-04 7.247468e-02
12 7.029877e-03 8.652390e-04 4.823035e-02
13 2.093581e-02 1.171646e-02 2.819237e-02
14 6.345086e-03 1.079784e-03 6.427316e-02
15 4.544313e-02 2.812027e-03 1.535693e-02
16 2.921416e-02 5.021341e-04 1.939577e-02
17 2.999495e-02 3.659268e-03 2.150808e-02
18 3.339192e-02 2.163098e-04 1.583683e-02
19 9.778044e-03 1.673780e-03 3.122071e-02
20 3.965679e-03 3.167367e-02 2.008536e-03
21 1.146539e-02 2.235201e-02 1.379724e-03
22 6.658668e-03 5.021341e-04 2.069363e-02
23 6.200880e-03 6.298740e-03 1.615198e-02
24 8.452590e-03 2.884130e-04 1.193761e-02
25 5.338932e-03 5.021341e-04 1.456486e-02
26 1.297859e-03 1.205759e-02 8.368901e-04
27 8.368901e-05 1.247386e-02 0.000000e+00
28 0.000000e+00 0.000000e+00 1.175283e-02heatmap(t(percT2))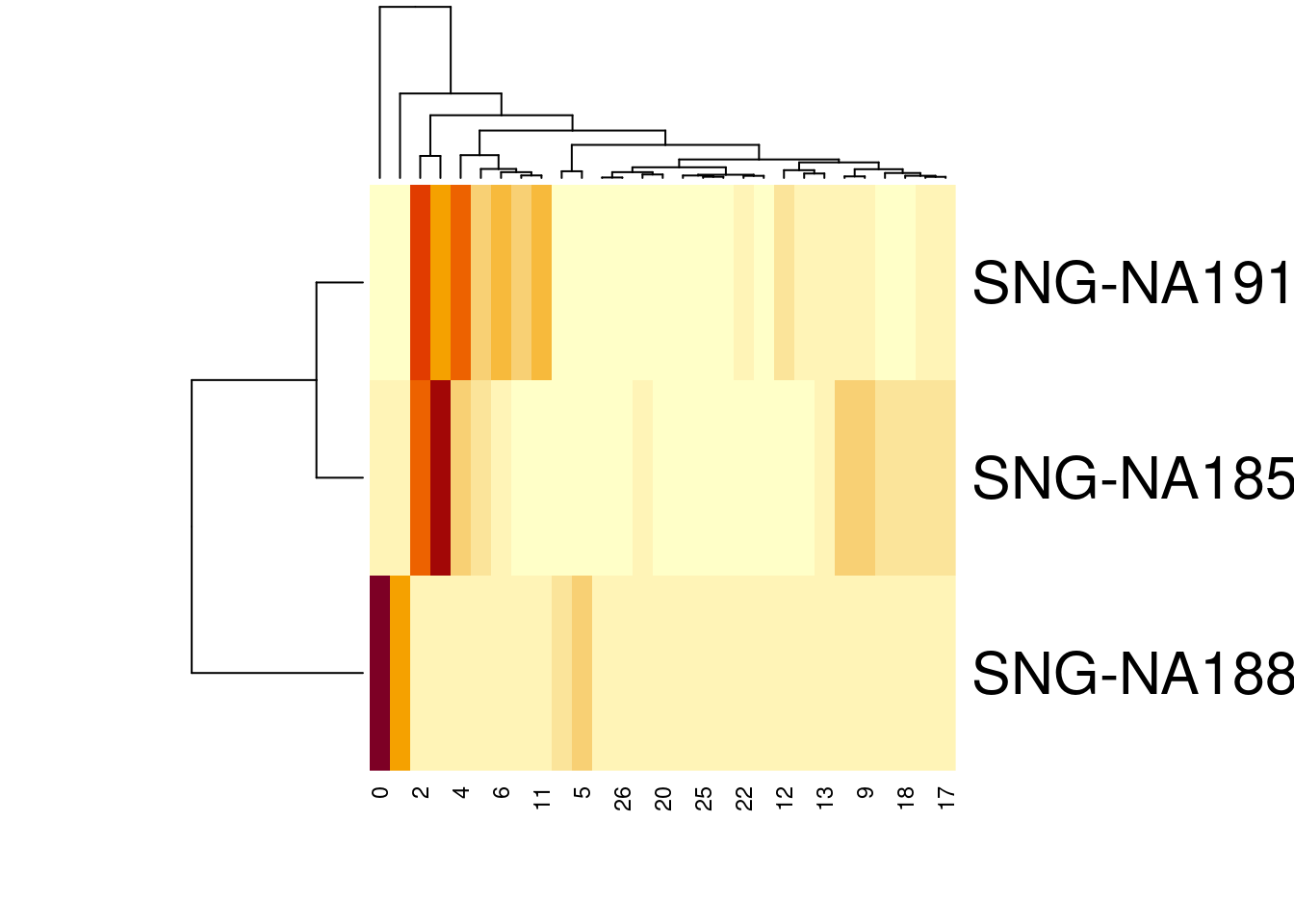
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
cormat<-round(cor(percT2),2)
library(reshape2)
melted_cormat<-melt(cormat)
ugly<-ggplot(data= melted_cormat, aes(x=Var1, y=Var2, fill=value)) +
geom_tile() +
ggtitle("Pairwise Pearson Correlation of the percent of cells from \neach cell line assigned to each Seurat Cluster")
get_lower_tri<- function(cormat){
cormat[upper.tri(cormat)]<-NA
return(cormat)
}
lower_tri<- get_lower_tri(cormat)
melted_tri<- melt(lower_tri)
pretty<-ggplot(data= melted_tri, aes(x=Var1, y=Var2, fill=value)) +
geom_tile(color="white") +
scale_fill_gradient2(low="blue", high="red", mid="white", midpoint = 0, limit= c(-1,1), space= "Lab", name="Pearson\nCorrelation") +
theme_minimal() +
ggtitle("Pairwise Pearson Correlation of the percent of cells from \neach cell line assigned to each Seurat Cluster")
pretty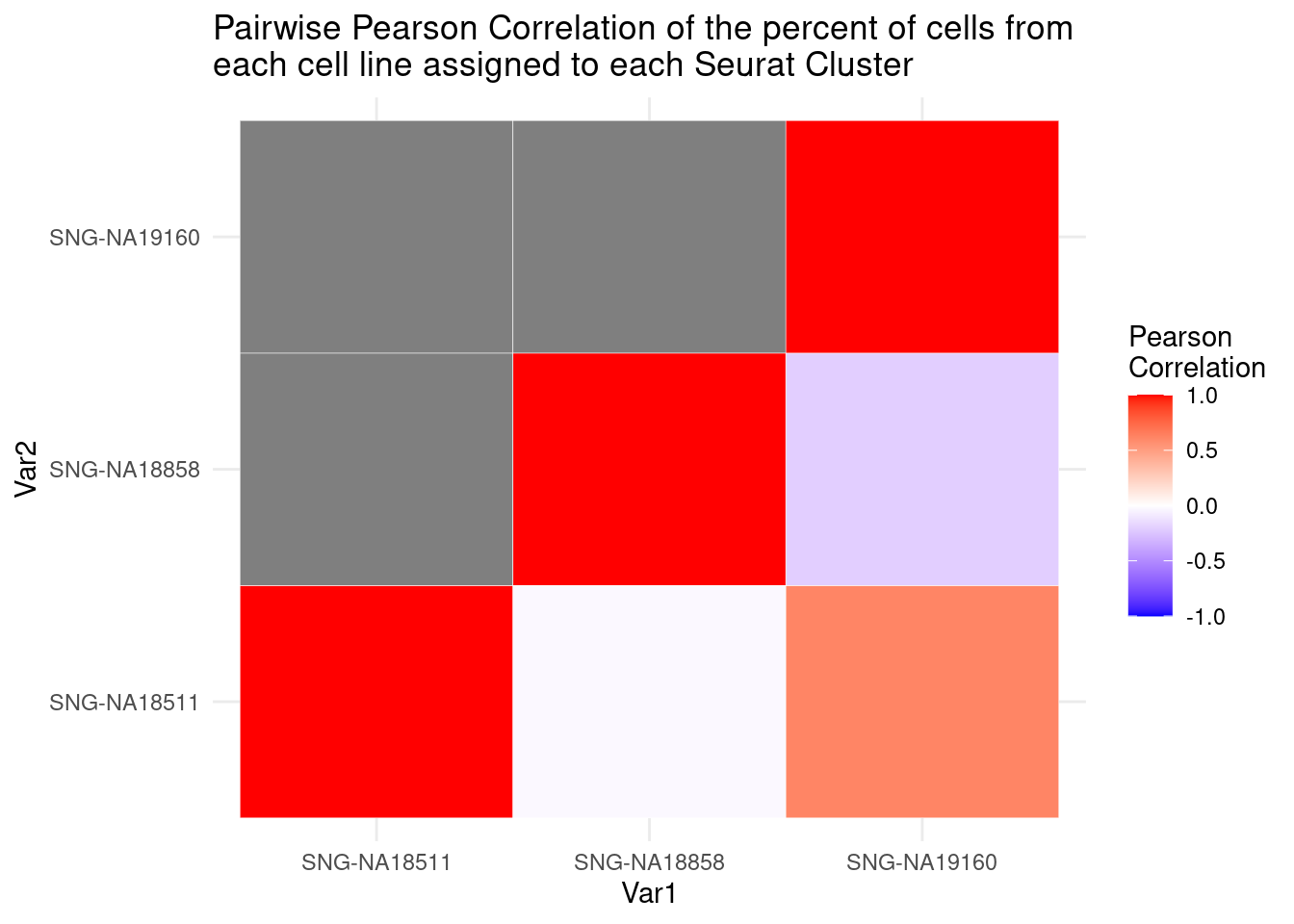
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#exploring similarity in the number of cells per individual between batches
merged.Batch1<- (subset(merged, Batch == "Batch1"))
b1t<- table(merged.Batch1$SCT_snn_res.1, merged.Batch1$individual)
remove("merged.Batch1")
b1tcolsums<- colSums(b1t)
percb1t<- b1t/b1tcolsums
merged.Batch2<- (subset(merged, Batch == "Batch2"))
b2t<- table(merged.Batch2$SCT_snn_res.1, merged.Batch2$individual)
remove("merged.Batch2")
b2tcolsums<- colSums(b2t)
percb2t<- b2t/b2tcolsums
merged.Batch3<- (subset(merged, Batch == "Batch3"))
b3t<- table(merged.Batch3$SCT_snn_res.1, merged.Batch3$individual)
remove("merged.Batch3")
b3tcolsums<- colSums(b3t)
percb3t<- b3t/b3tcolsumscols1<- c("Batch1_18511","Batch1_18858","Batch1_19160", "Batch2_18511", "Batch2_18858","Batch2_19160",
"Batch3_18511","Batch3_18858", "Batch3_19160")
cols2<- c("Batch1_18511", "Batch2_18511", "Batch3_18511","Batch1_18858", "Batch2_18858", "Batch3_18858","Batch1_19160", "Batch2_19160", "Batch3_19160")
fullpercs<- as.data.frame(cbind(percb1t[,1:3], percb2t,percb3t))
colnames(fullpercs)<-cols1
fullpercs<- cbind(fullpercs$Batch1_18511, fullpercs$Batch2_18511, fullpercs$Batch3_18511,
fullpercs$Batch1_18858, fullpercs$Batch2_18858, fullpercs$Batch3_18858,
fullpercs$Batch1_19160, fullpercs$Batch2_19160, fullpercs$Batch3_19160)
colnames(fullpercs)<-cols2
fullpercs_cor<- round(cor(fullpercs),2)
fullpercs_melt<- melt(fullpercs_cor)
ggplot(data= fullpercs_melt, aes(x=Var1, y=Var2, fill=value)) +
geom_tile(color="white") +
scale_fill_gradient2(low="blue", high="red", mid="white", midpoint = 0, limit= c(-1,1), space= "Lab", name="Pearson\nCorrelation") +
theme_minimal() +
theme(axis.text.x = element_text(angle = 30, hjust=1))+
ggtitle("Pairwise Pearson Correlation of the percent of cells from \neach cell line assigned to each Seurat Cluster (res 1)")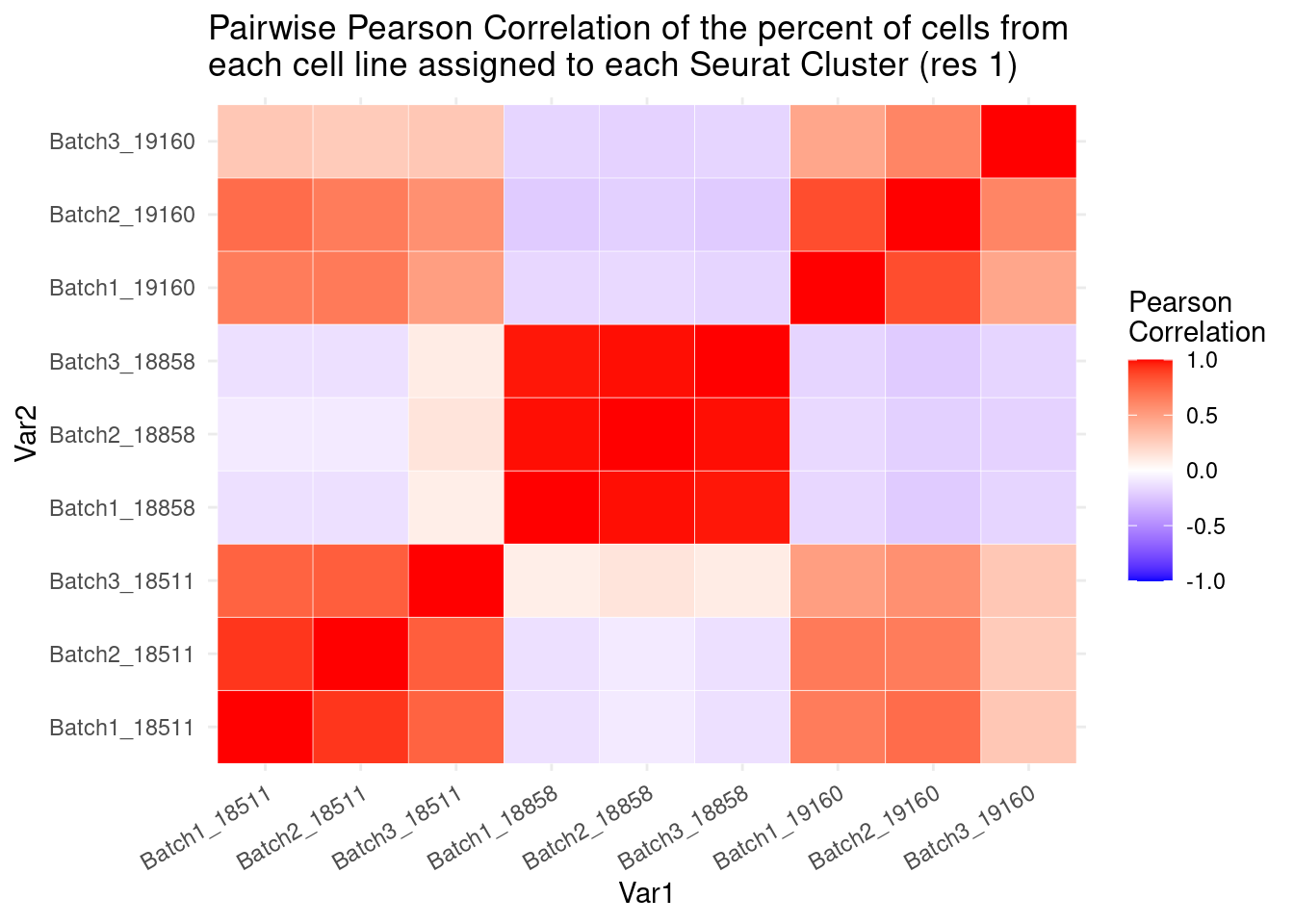
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#now clustering individual_Batch samples with hierarchical clustering/they will get reordered based on similarity
beauty<- colorRampPalette(brewer.pal(9,"Purples"))(200)
rownames(fullpercs)<- c(0:(nrow(fullpercs)-1))
heatmap(as.matrix(fullpercs), scale="none", col=beauty, cexCol = .7, cexRow=.6)
text(1:ncol(fullpercs),labels=names(fullpercs),srt=30)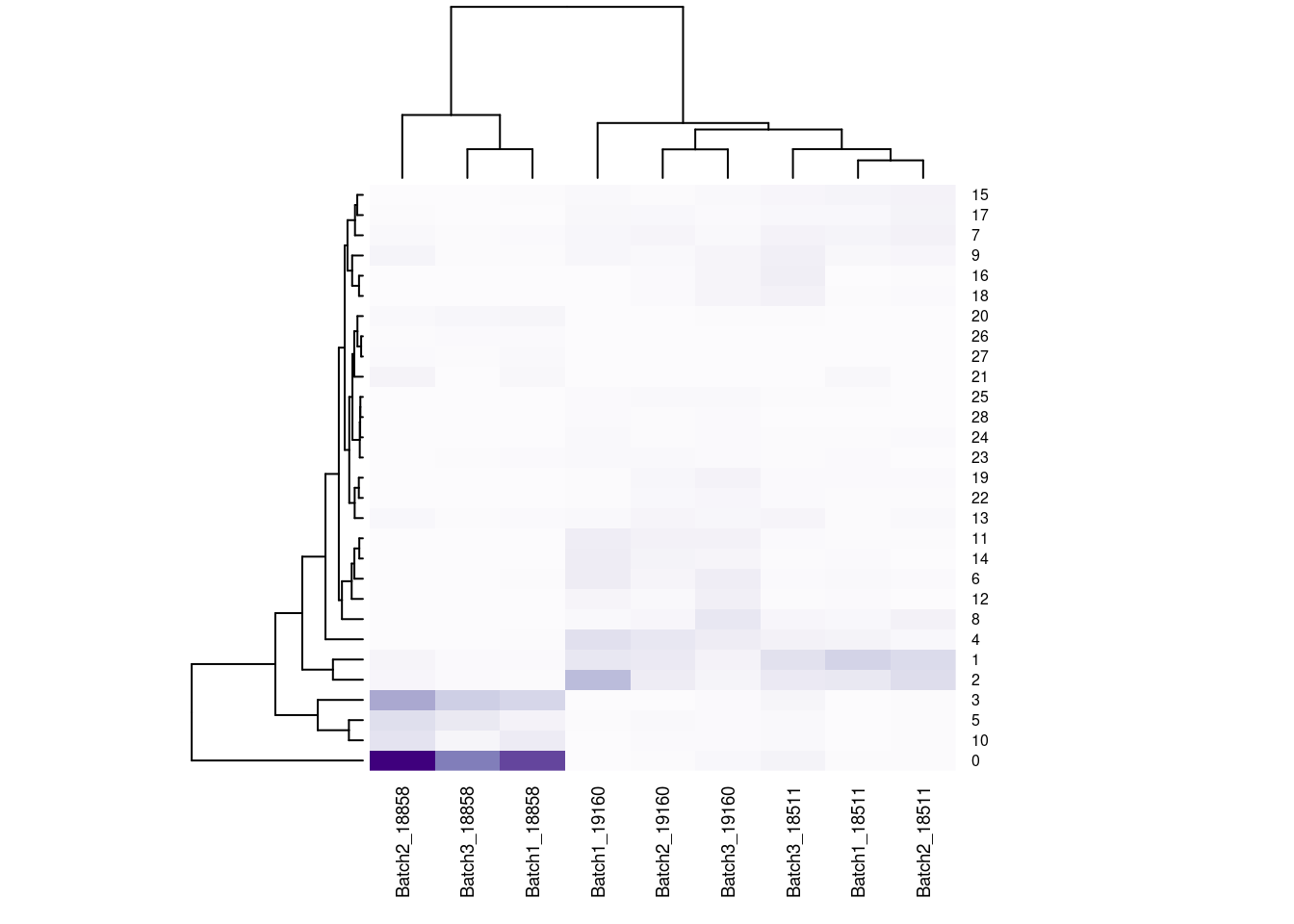
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#generate a heatmap of the proportion of cells from each individual_batch in each seurat cluster. dendrograms based on similarity of the vectors. should be colored by the value(proportion), but some of the cluster/sample values to seem to match with the colorReclustering with less resolution, check if everything is robust
#reassign idents
Idents(merged)<- 'SCT_snn_res.0.5'DimPlot(merged, reduction = "umap")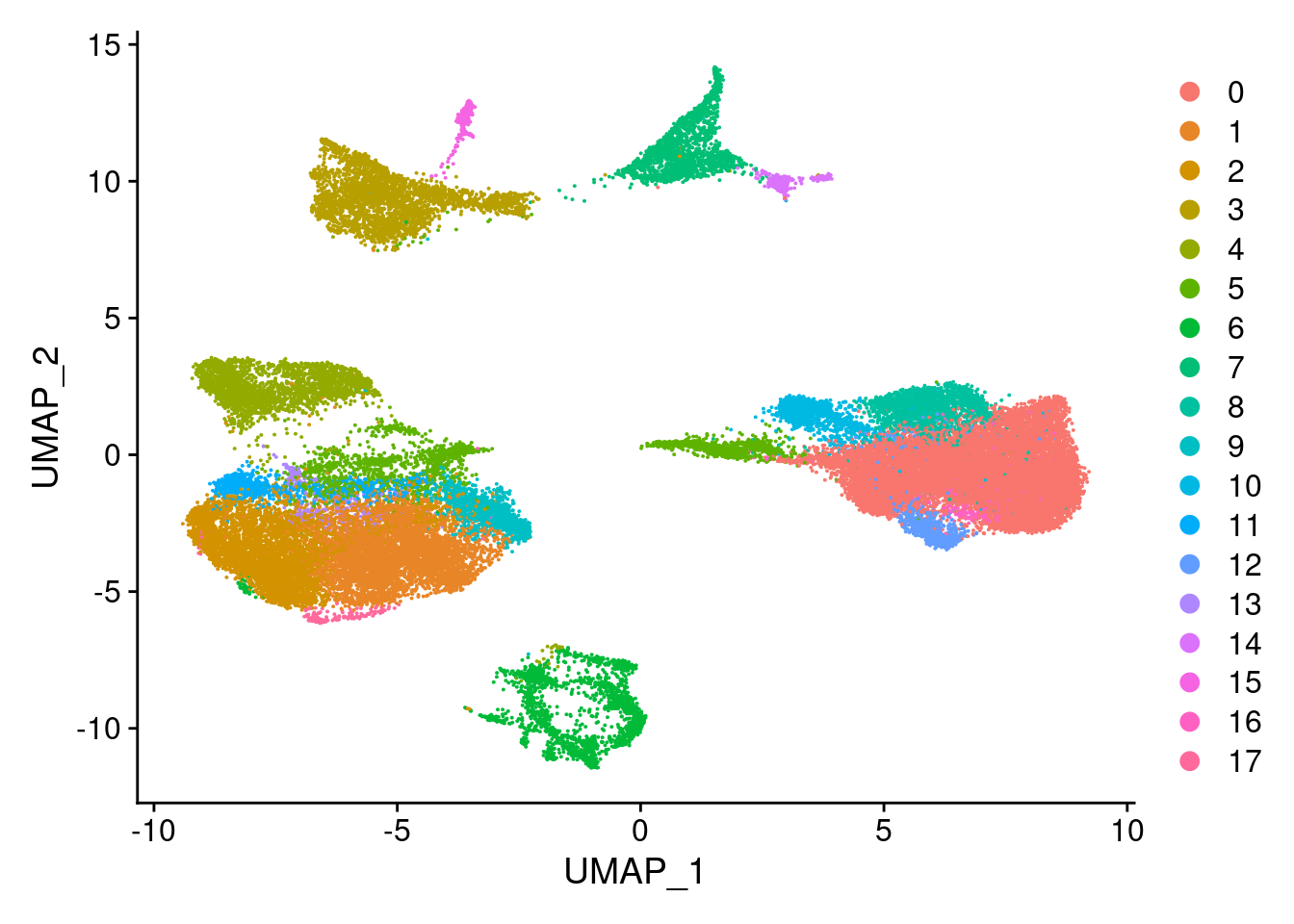
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap", group.by = "Batch")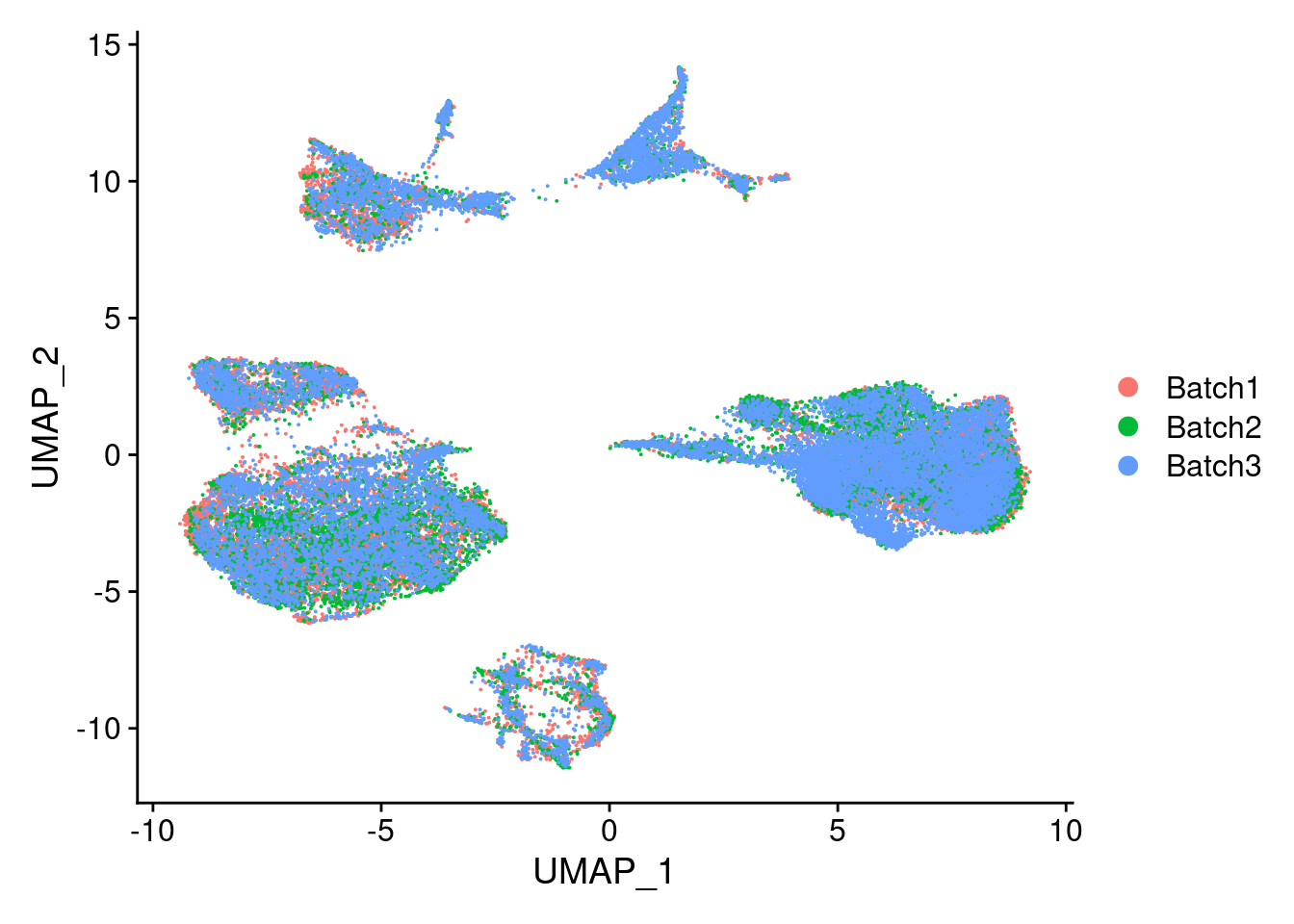
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap", group.by = "individual")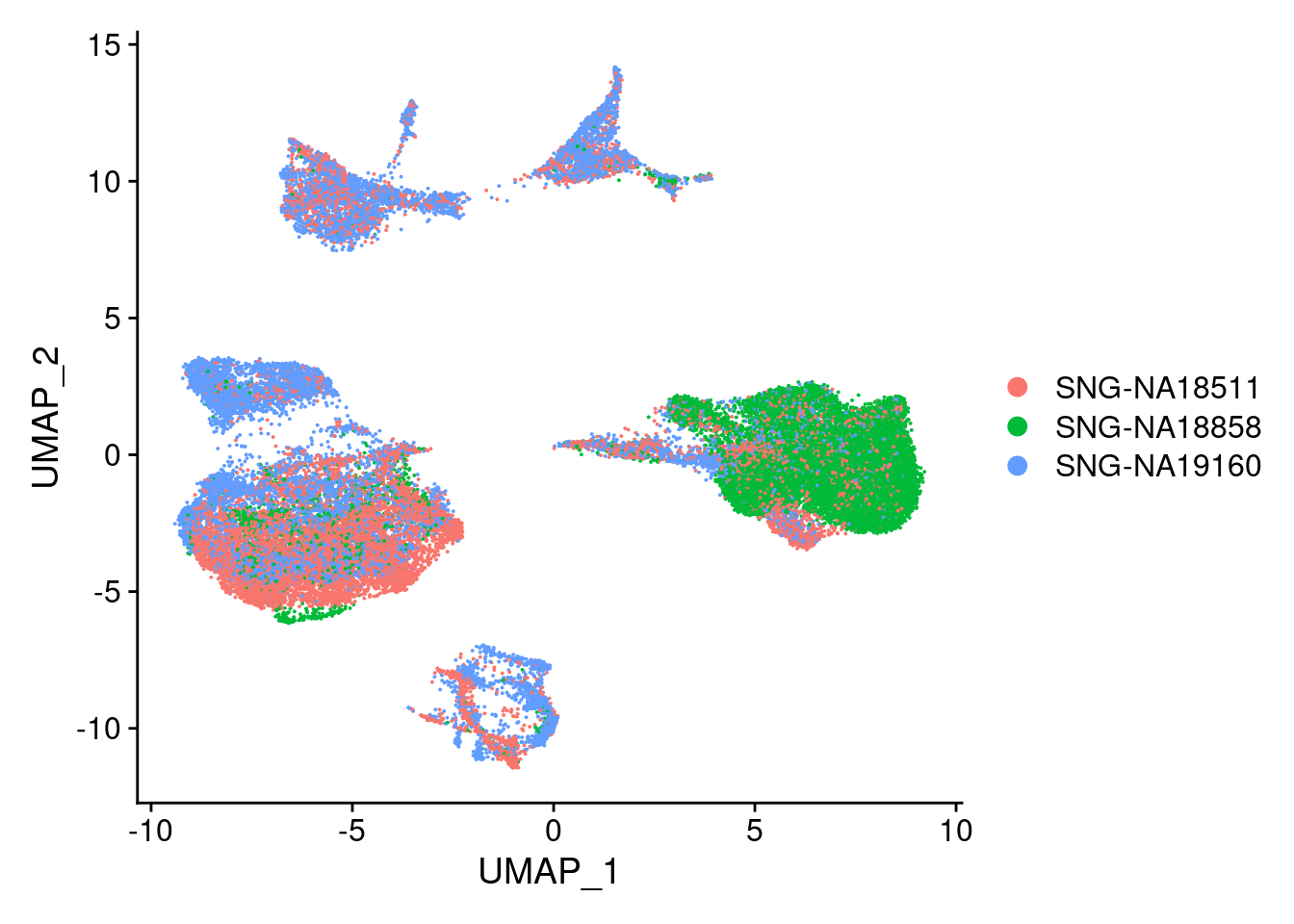
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
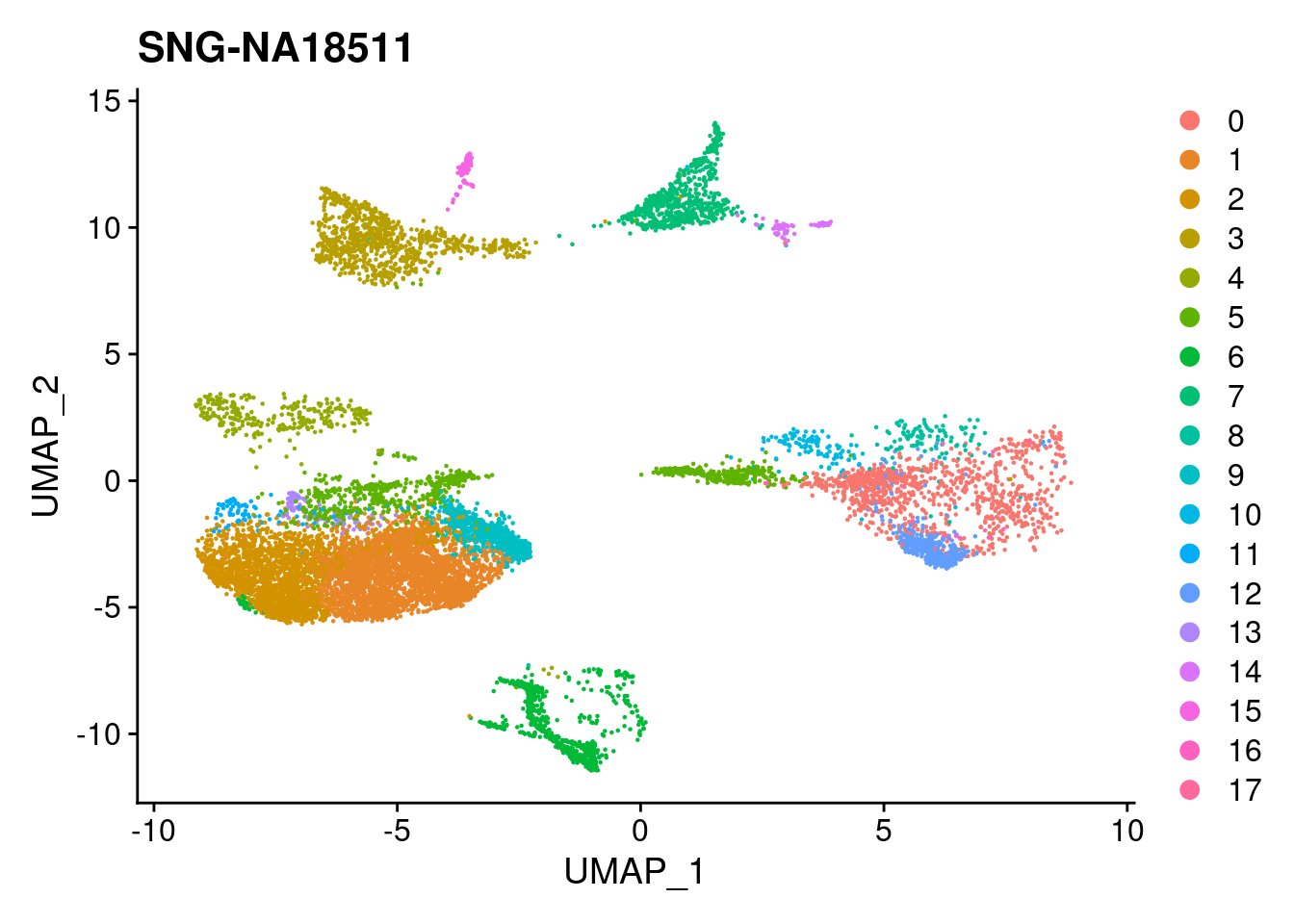
xlim <- c(min(merged@reductions$umap@cell.embeddings[,'UMAP_1']),
max(merged@reductions$umap@cell.embeddings[,'UMAP_1']))
ylim <- c(min(merged@reductions$umap@cell.embeddings[,'UMAP_2']),
max(merged@reductions$umap@cell.embeddings[,'UMAP_2']))
for (i in individuals)
{
print(DimPlot(merged, reduction = "umap",
cells = WhichCells(merged, expression = individual == i)) +
xlim(xlim) + ylim(ylim) + ggtitle(i))
}
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#exploring similarity in the number of cells per individual between batches
merged.Batch1<- (subset(merged, Batch == "Batch1"))
b1t<- table(merged.Batch1$SCT_snn_res.0.5, merged.Batch1$individual)
remove("merged.Batch1")
b1tcolsums<- colSums(b1t)
percb1t<- b1t/b1tcolsums
merged.Batch2<- (subset(merged, Batch == "Batch2"))
b2t<- table(merged.Batch2$SCT_snn_res.0.5, merged.Batch2$individual)
remove("merged.Batch2")
b2tcolsums<- colSums(b2t)
percb2t<- b2t/b2tcolsums
merged.Batch3<- (subset(merged, Batch == "Batch3"))
b3t<- table(merged.Batch3$SCT_snn_res.0.5, merged.Batch3$individual)
remove("merged.Batch3")
b3tcolsums<- colSums(b3t)
percb3t<- b3t/b3tcolsumscols1<- c("Batch1_18511","Batch1_18858","Batch1_19160", "Batch2_18511", "Batch2_18858","Batch2_19160",
"Batch3_18511","Batch3_18858", "Batch3_19160")
cols2<- c("Batch1_18511", "Batch2_18511", "Batch3_18511","Batch1_18858", "Batch2_18858", "Batch3_18858","Batch1_19160", "Batch2_19160", "Batch3_19160")
fullpercs<- as.data.frame(cbind(percb1t[,1:3], percb2t,percb3t))
colnames(fullpercs)<-cols1
fullpercs<- cbind(fullpercs$Batch1_18511, fullpercs$Batch2_18511, fullpercs$Batch3_18511,
fullpercs$Batch1_18858, fullpercs$Batch2_18858, fullpercs$Batch3_18858,
fullpercs$Batch1_19160, fullpercs$Batch2_19160, fullpercs$Batch3_19160)
colnames(fullpercs)<-cols2
fullpercs_cor<- round(cor(fullpercs),2)
fullpercs_melt<- melt(fullpercs_cor)
ggplot(data= fullpercs_melt, aes(x=Var1, y=Var2, fill=value)) +
geom_tile(color="white") +
scale_fill_gradient2(low="blue", high="red", mid="white", midpoint = 0, limit= c(-1,1), space= "Lab", name="Pearson\nCorrelation") +
theme_minimal() +
theme(axis.text.x = element_text(angle = 30, hjust=1))+
ggtitle("Pairwise Pearson Correlation of the percent of cells from \neach cell line assigned to each Seurat Cluster\ncluster res. 0.5")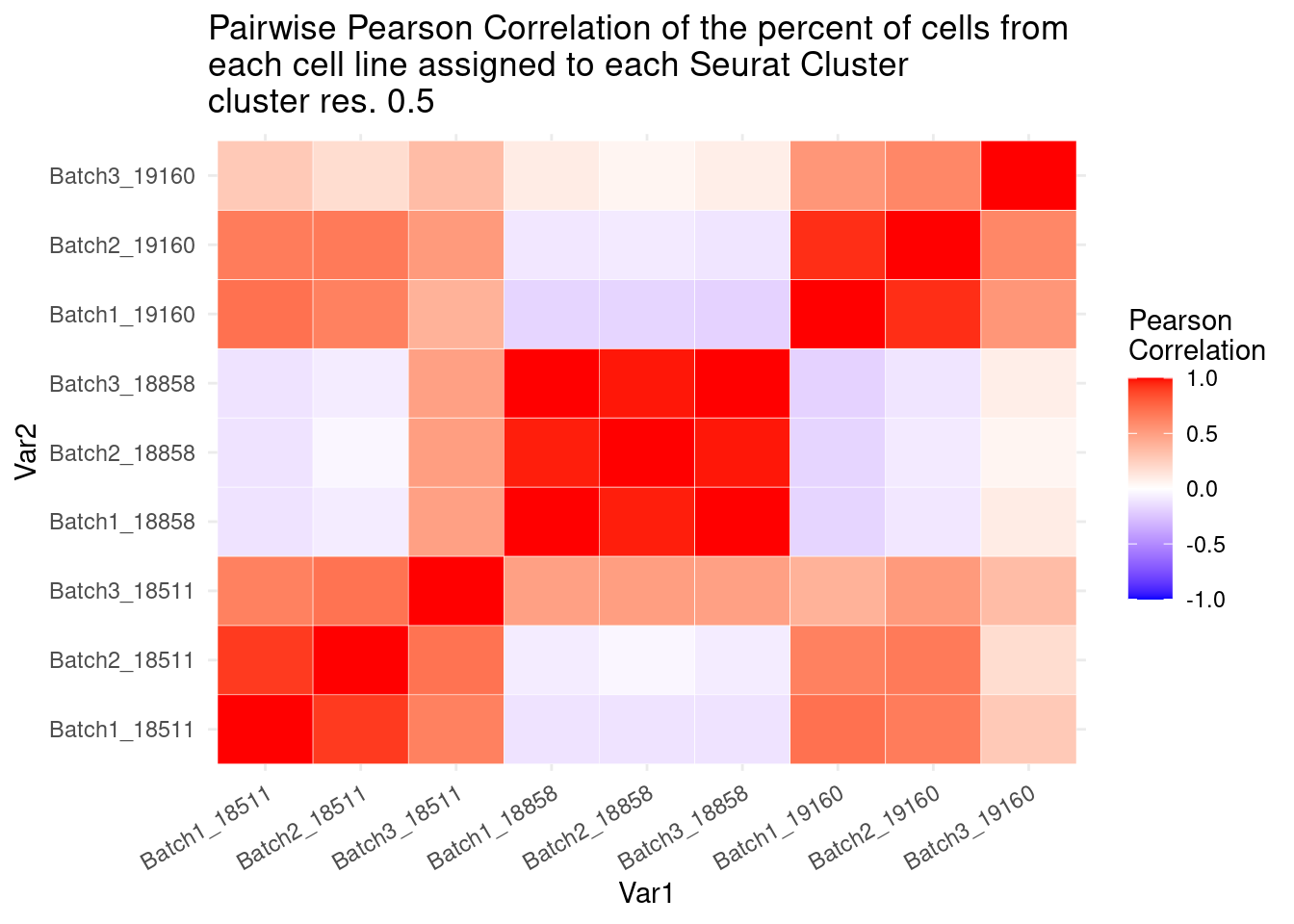
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#now clustering individual_Batch samples with hierarchical clustering/they will get reordered based on similarity
beauty<- colorRampPalette(brewer.pal(9,"Purples"))(200)
rownames(fullpercs)<- c(0:(nrow(fullpercs)-1))
heatmap(as.matrix(fullpercs), scale="none", col=beauty, cexCol = .7, cexRow=.6)
text(1:ncol(fullpercs),labels=names(fullpercs),srt=30)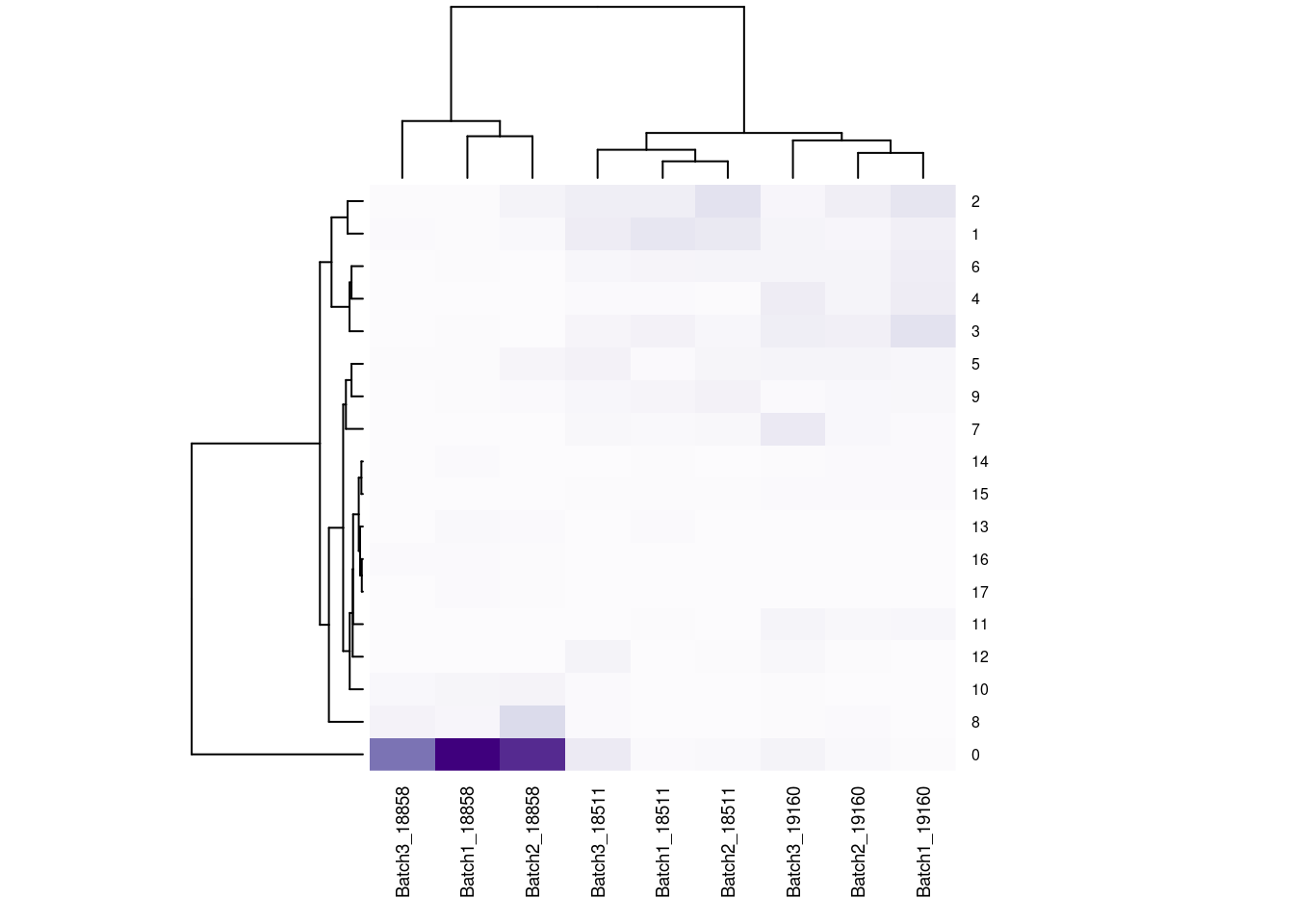
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#generate a heatmap of the raw proportion of cells from each individual_batch in each seurat cluster. dendrograms based on similarity of the vectors. should be colored by the value(proportion), but some of the cluster/sample values to seem to match with the color#reassign idents
Idents(merged)<- 'SCT_snn_res.0.1'DimPlot(merged, reduction = "umap")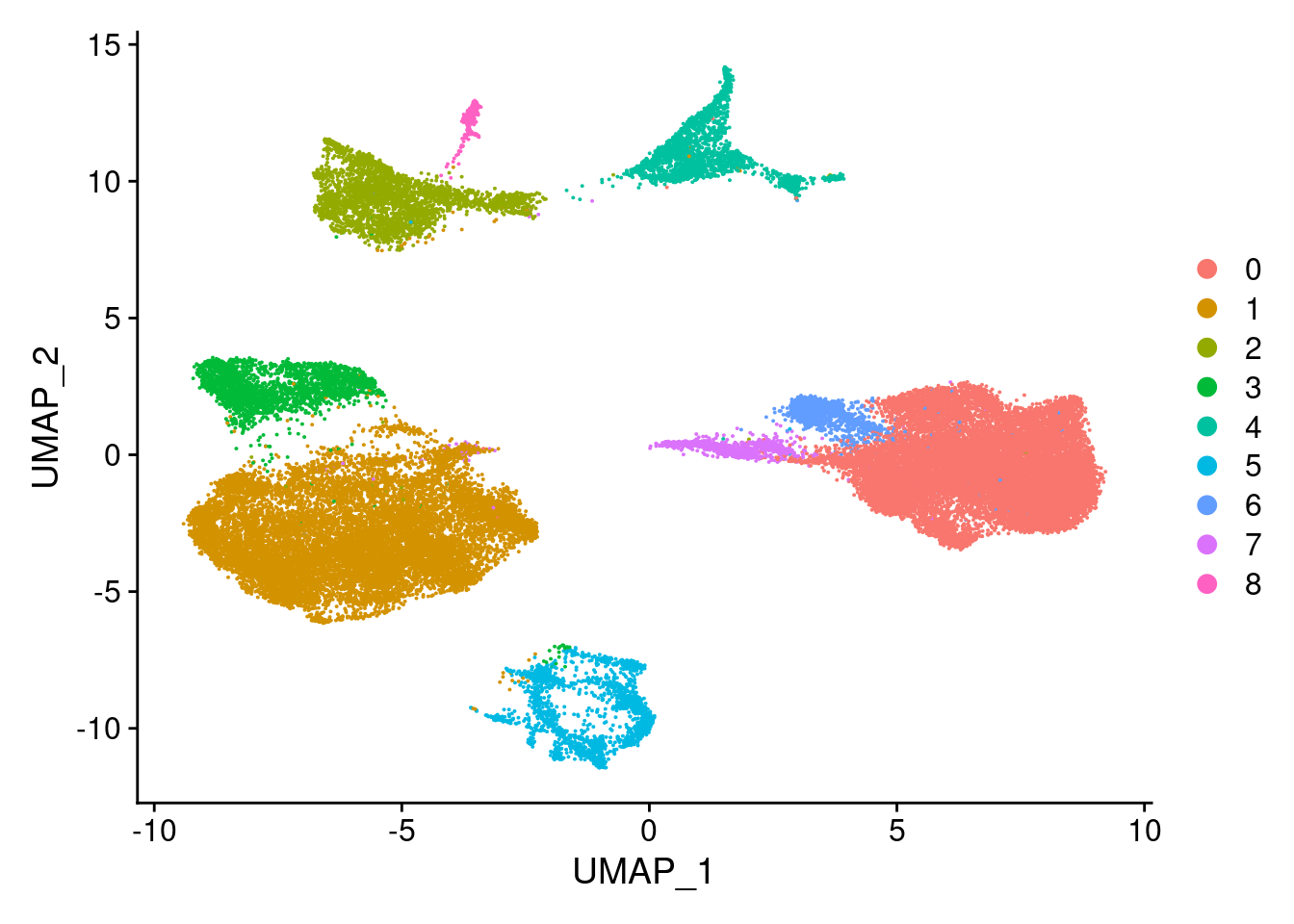
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap", group.by = "Batch")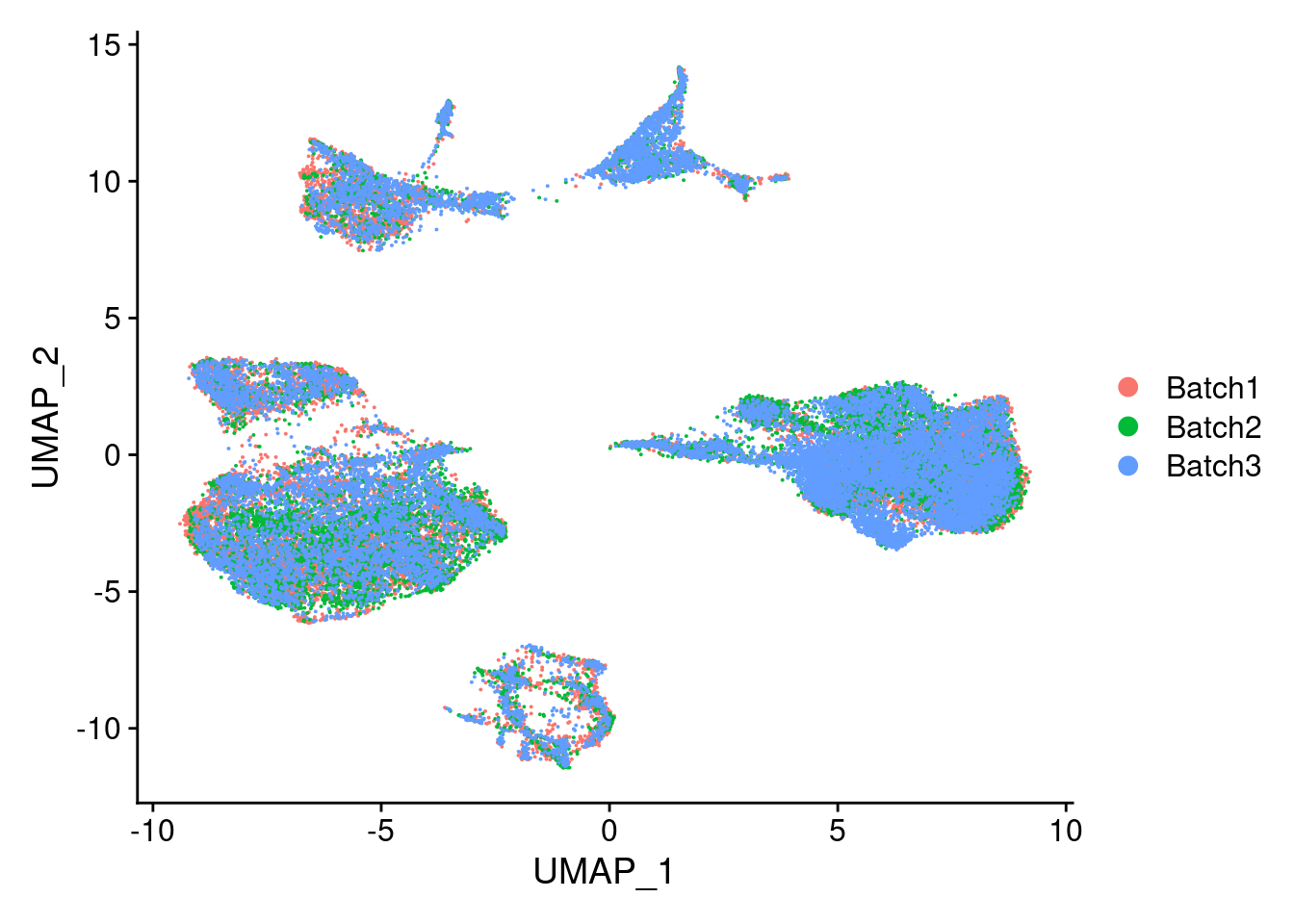
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap", group.by = "individual")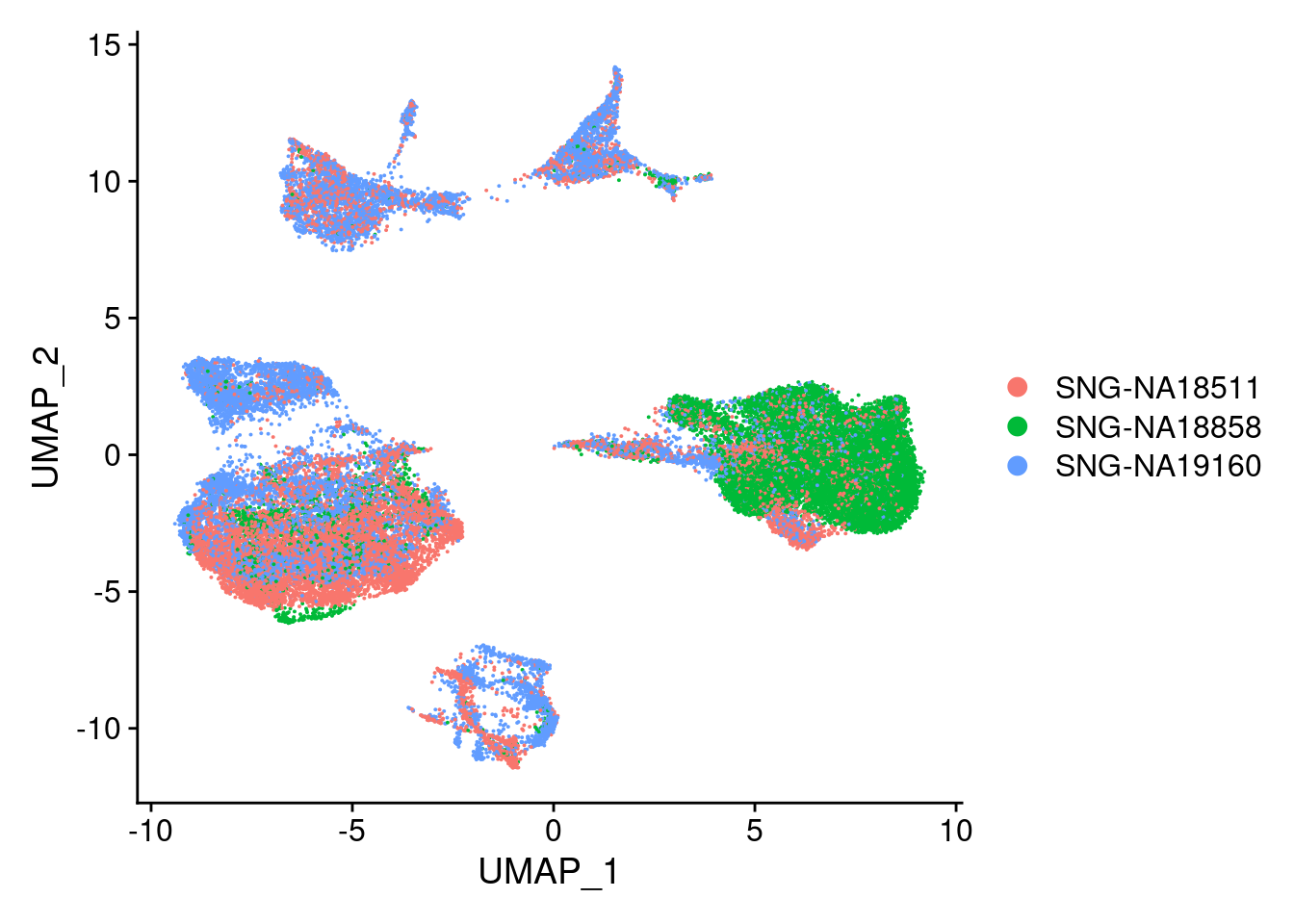
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
xlim <- c(min(merged@reductions$umap@cell.embeddings[,'UMAP_1']),
max(merged@reductions$umap@cell.embeddings[,'UMAP_1']))
ylim <- c(min(merged@reductions$umap@cell.embeddings[,'UMAP_2']),
max(merged@reductions$umap@cell.embeddings[,'UMAP_2']))
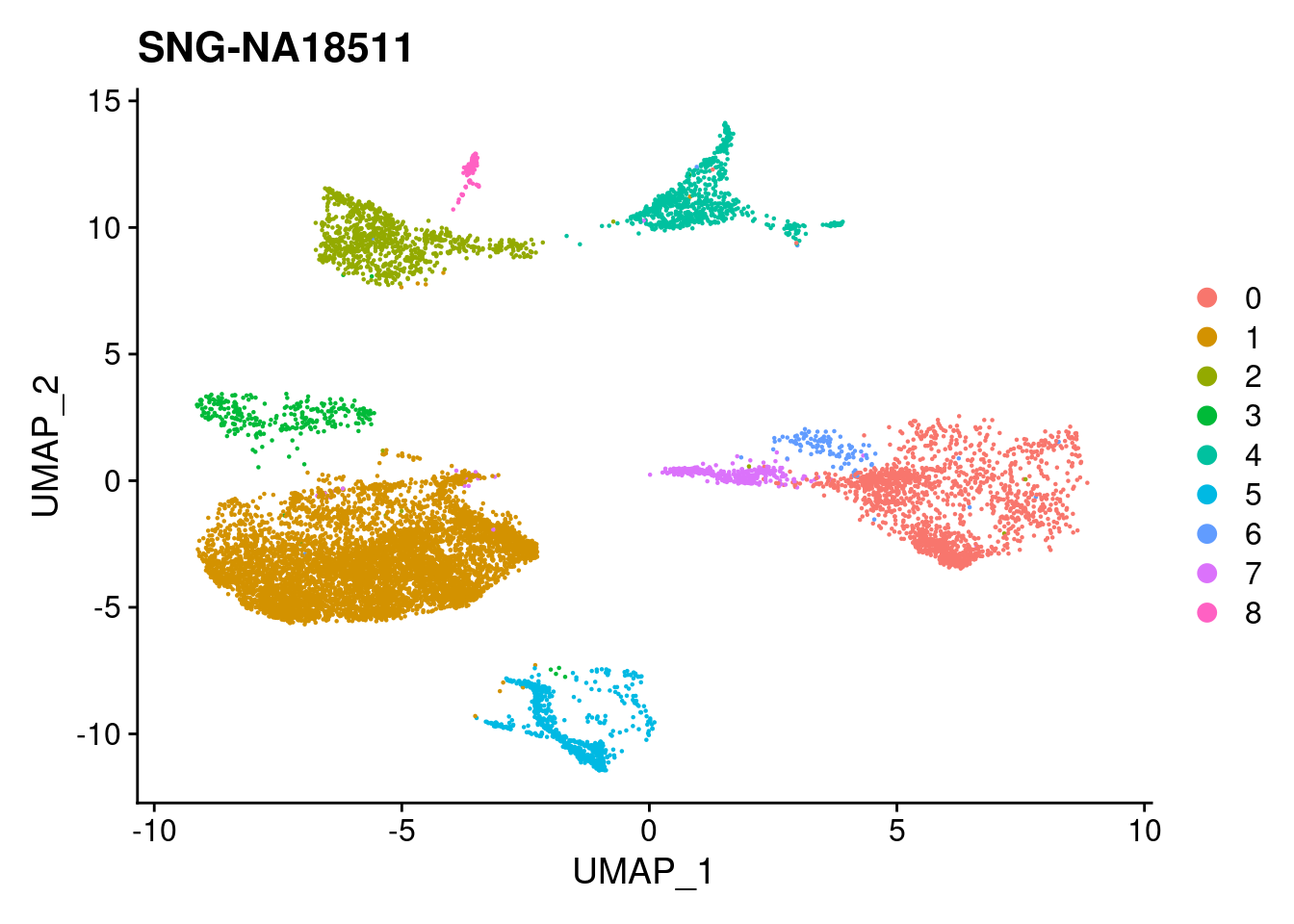
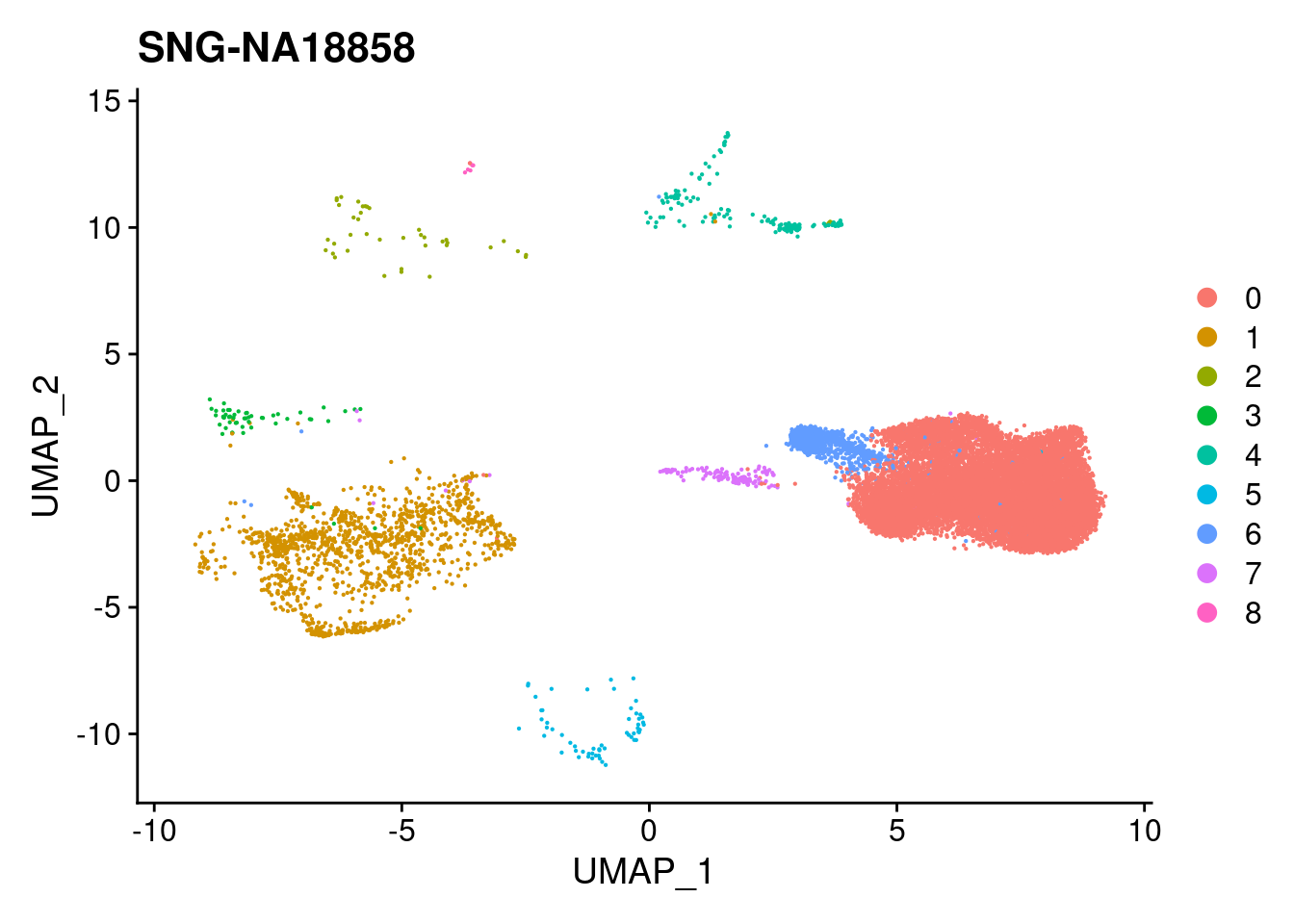
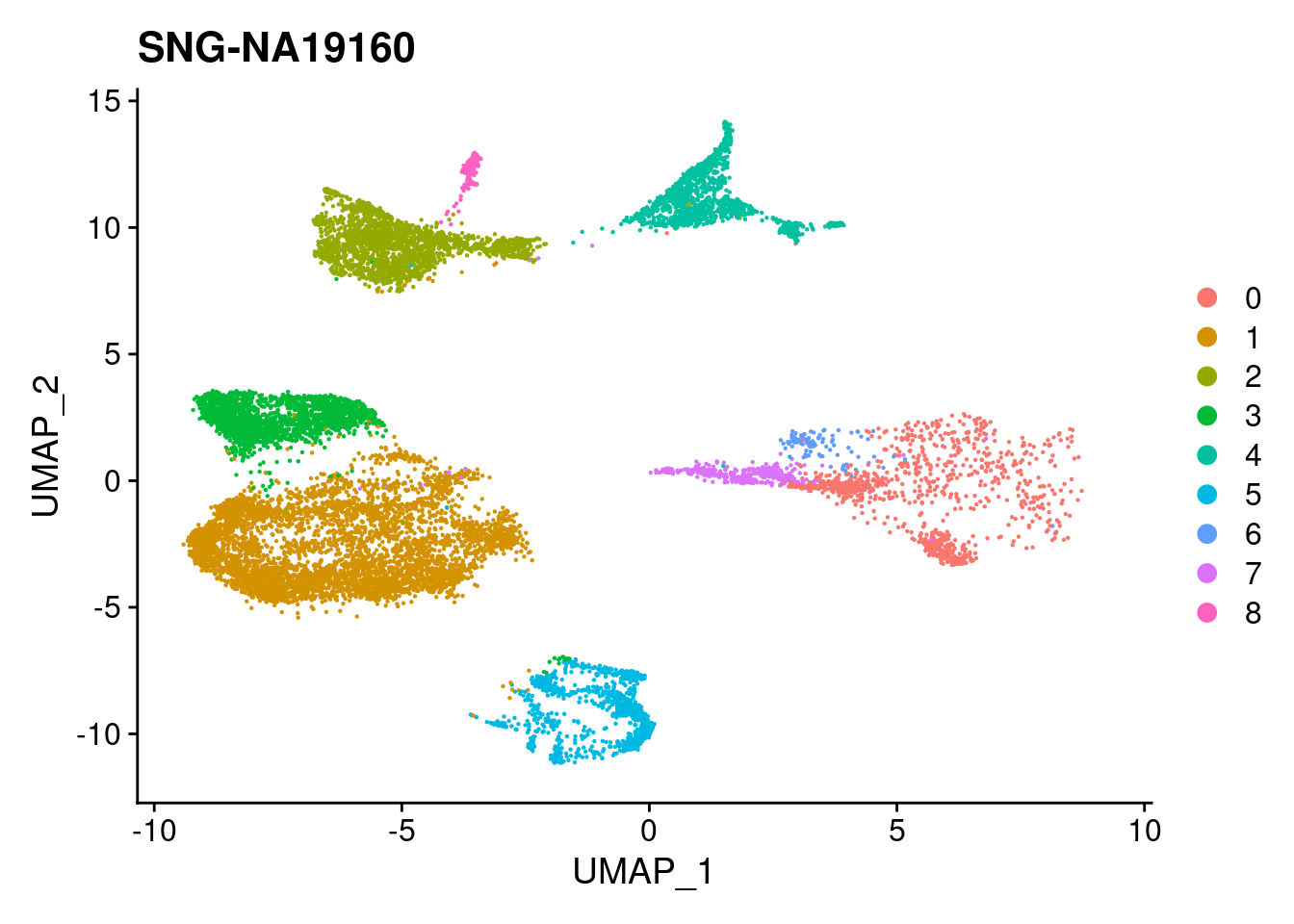
for (i in individuals)
{
print(DimPlot(merged, reduction = "umap",
cells = WhichCells(merged, expression = individual == i)) +
xlim(xlim) + ylim(ylim) + ggtitle(i))
}
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#exploring similarity in the number of cells per individual between batches
merged.Batch1<- (subset(merged, Batch == "Batch1"))
b1t<- table(merged.Batch1$SCT_snn_res.0.1, merged.Batch1$individual)
remove("merged.Batch1")
b1tcolsums<- colSums(b1t)
percb1t<- b1t/b1tcolsums
merged.Batch2<- (subset(merged, Batch == "Batch2"))
b2t<- table(merged.Batch2$SCT_snn_res.0.1, merged.Batch2$individual)
remove("merged.Batch2")
b2tcolsums<- colSums(b2t)
percb2t<- b2t/b2tcolsums
merged.Batch3<- (subset(merged, Batch == "Batch3"))
b3t<- table(merged.Batch3$SCT_snn_res.0.1, merged.Batch3$individual)
remove("merged.Batch3")
b3tcolsums<- colSums(b3t)
percb3t<- b3t/b3tcolsumscols1<- c("Batch1_18511","Batch1_18858","Batch1_19160", "Batch2_18511", "Batch2_18858","Batch2_19160",
"Batch3_18511","Batch3_18858", "Batch3_19160")
cols2<- c("Batch1_18511", "Batch2_18511", "Batch3_18511","Batch1_18858", "Batch2_18858", "Batch3_18858","Batch1_19160", "Batch2_19160", "Batch3_19160")
fullpercs<- as.data.frame(cbind(percb1t[,1:3], percb2t,percb3t))
colnames(fullpercs)<-cols1
fullpercs<- cbind(fullpercs$Batch1_18511, fullpercs$Batch2_18511, fullpercs$Batch3_18511,
fullpercs$Batch1_18858, fullpercs$Batch2_18858, fullpercs$Batch3_18858,
fullpercs$Batch1_19160, fullpercs$Batch2_19160, fullpercs$Batch3_19160)
colnames(fullpercs)<-cols2
fullpercs_cor<- round(cor(fullpercs),2)
fullpercs_melt<- melt(fullpercs_cor)
ggplot(data= fullpercs_melt, aes(x=Var1, y=Var2, fill=value)) +
geom_tile(color="white") +
scale_fill_gradient2(low="blue", high="red", mid="white", midpoint = 0, limit= c(-1,1), space= "Lab", name="Pearson\nCorrelation") +
theme_minimal() +
theme(axis.text.x = element_text(angle = 30, hjust=1))+
ggtitle("Pairwise Pearson Correlation of the percent of cells from \neach cell line assigned to each Seurat Cluster\ncluster res. 0.1")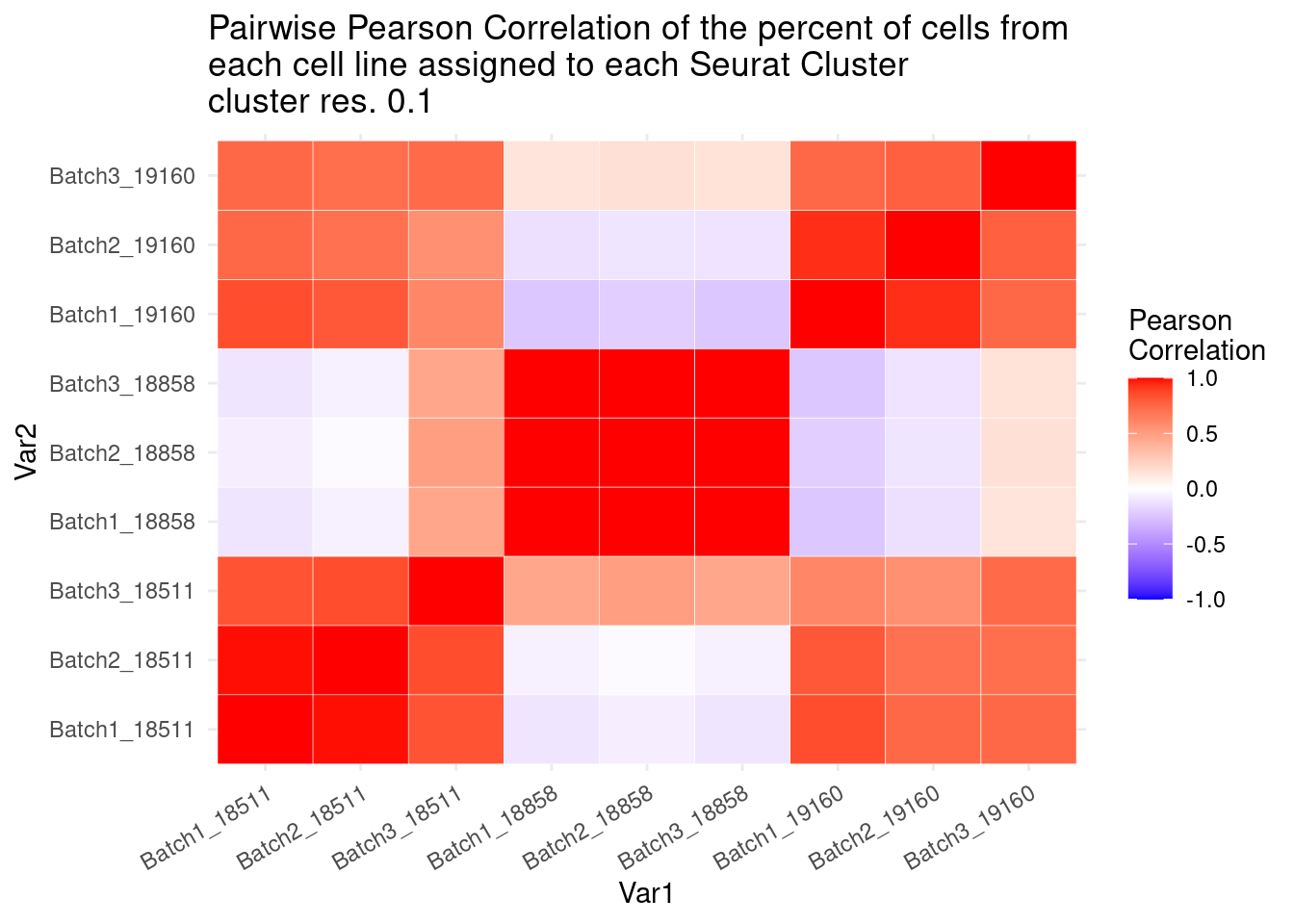
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#now clustering individual_Batch samples with hierarchical clustering/they will get reordered based on similarity
beauty<- colorRampPalette(brewer.pal(9,"Purples"))(200)
#fullnonorm<- as.data.frame(cbind(b1t[,1:3], b2t,b3t))
#colnames(fullnonorm)<-cols1
#heatmap((as.matrix(fullnonorm)), scale="column", col= beauty)
rownames(fullpercs)<- c(0:(nrow(fullpercs)-1))
heatmap(as.matrix(fullpercs), scale="none", col=beauty, cexCol = .7, cexRow=.6)
text(1:ncol(fullpercs),labels=names(fullpercs),srt=30)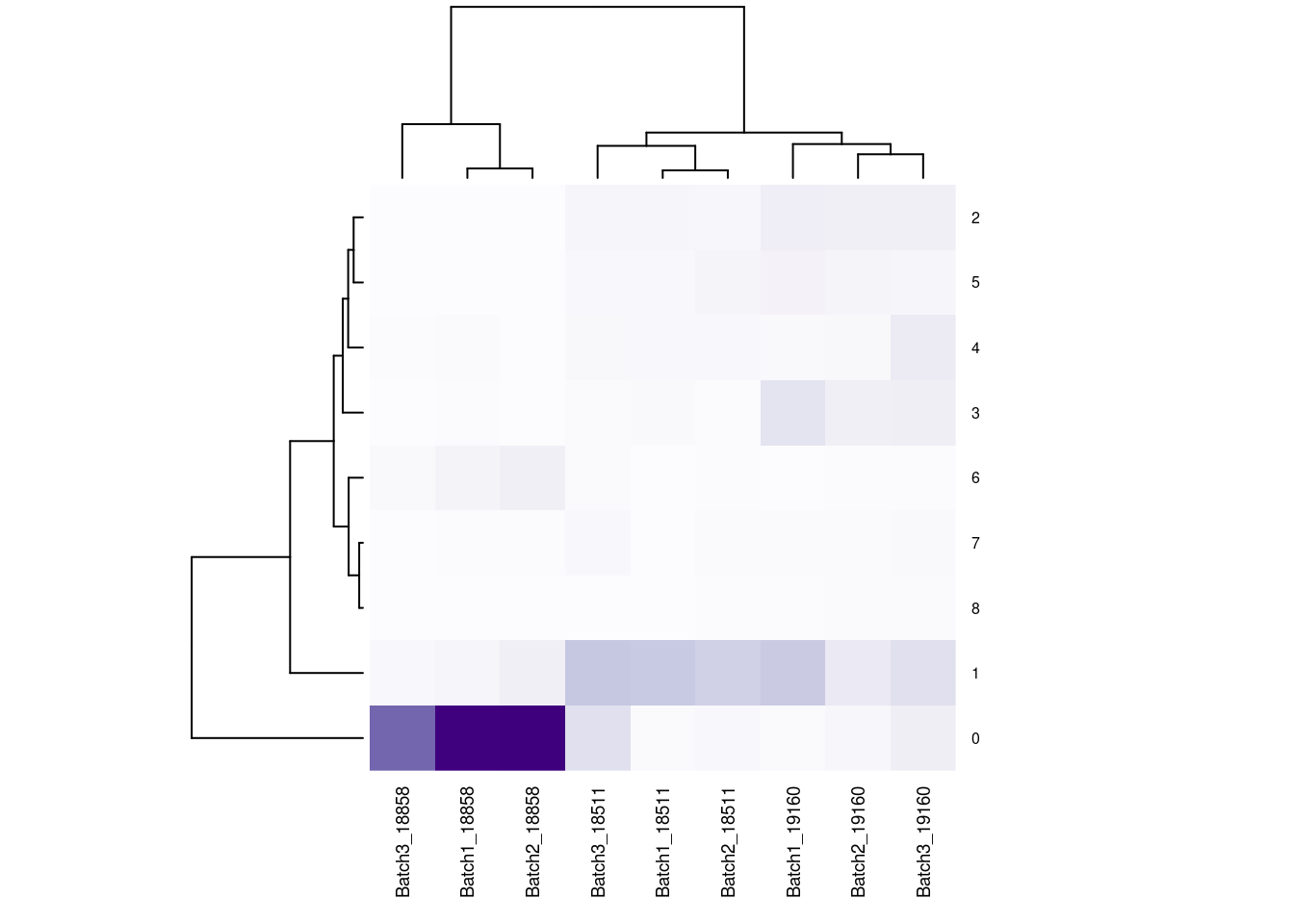
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#generate a heatmap of the raw proportion of cells from each individual_batch in each seurat cluster. dendrograms based on similarity of the vectors. should be colored by the value(proportion), but some of the cluster/sample values to seem to match with the color#reassign idents
Idents(merged)<- 'SCT_snn_res.0.8'DimPlot(merged, reduction = "umap")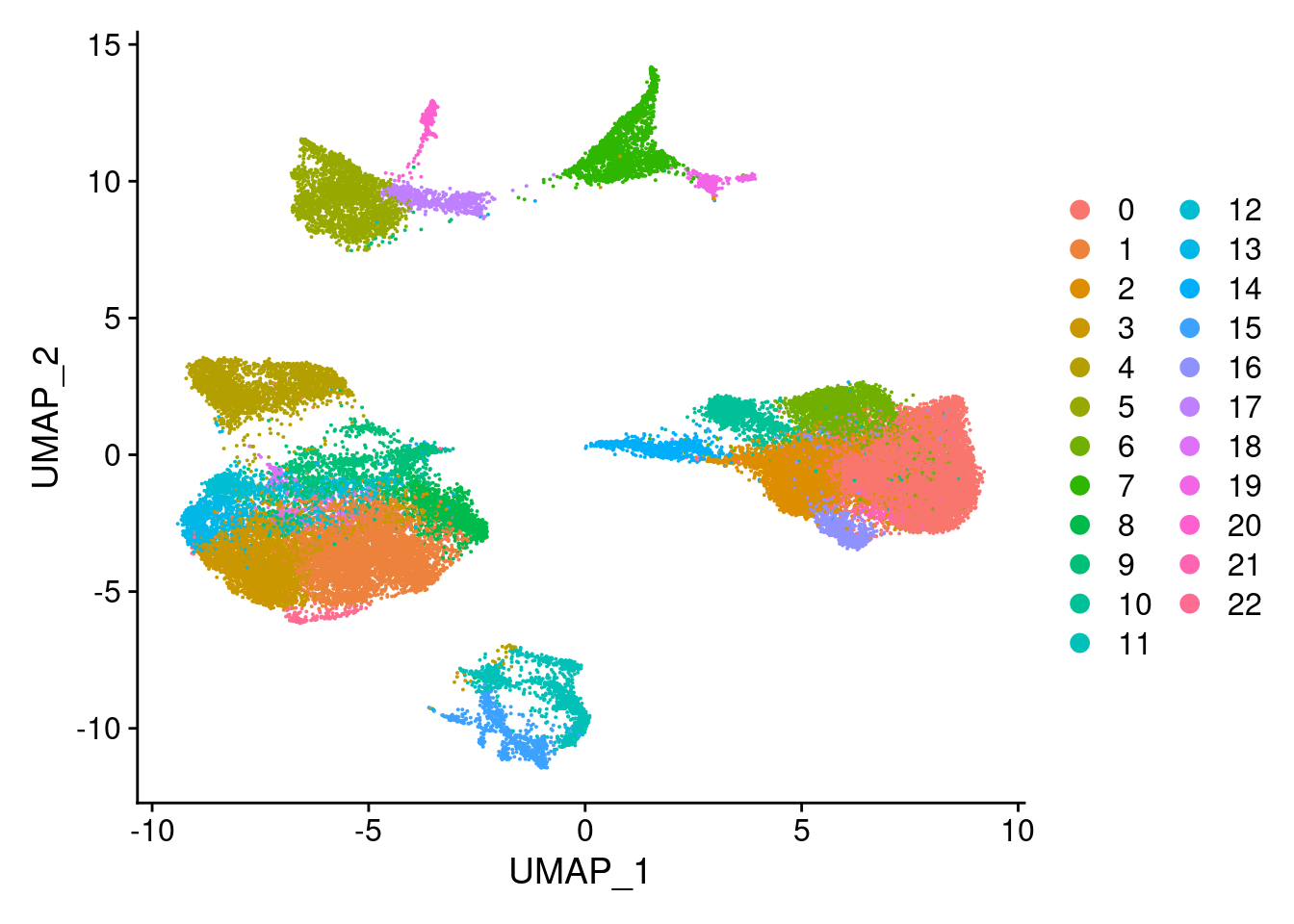
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap", group.by = "Batch")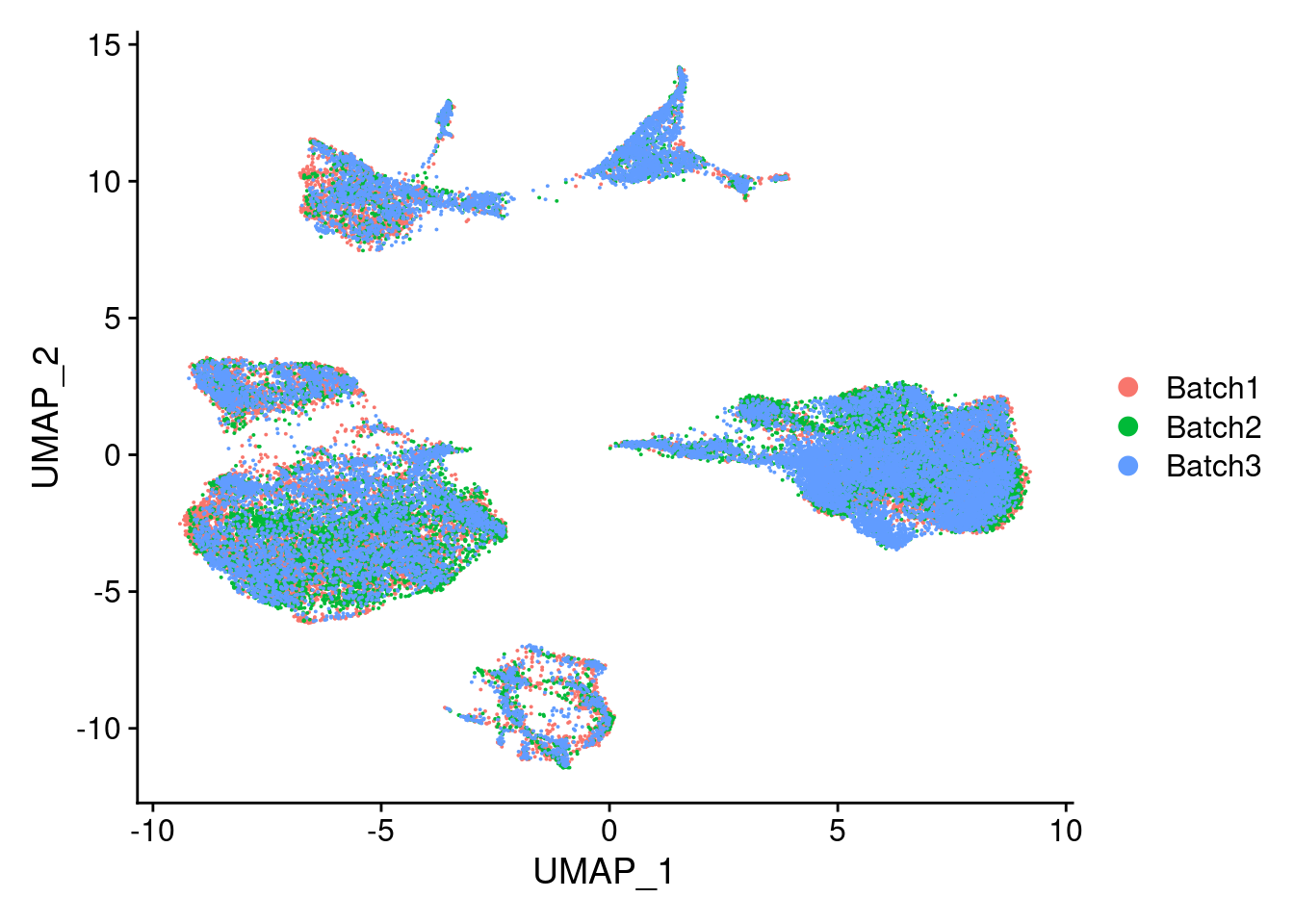
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
DimPlot(merged, reduction = "umap", group.by = "individual")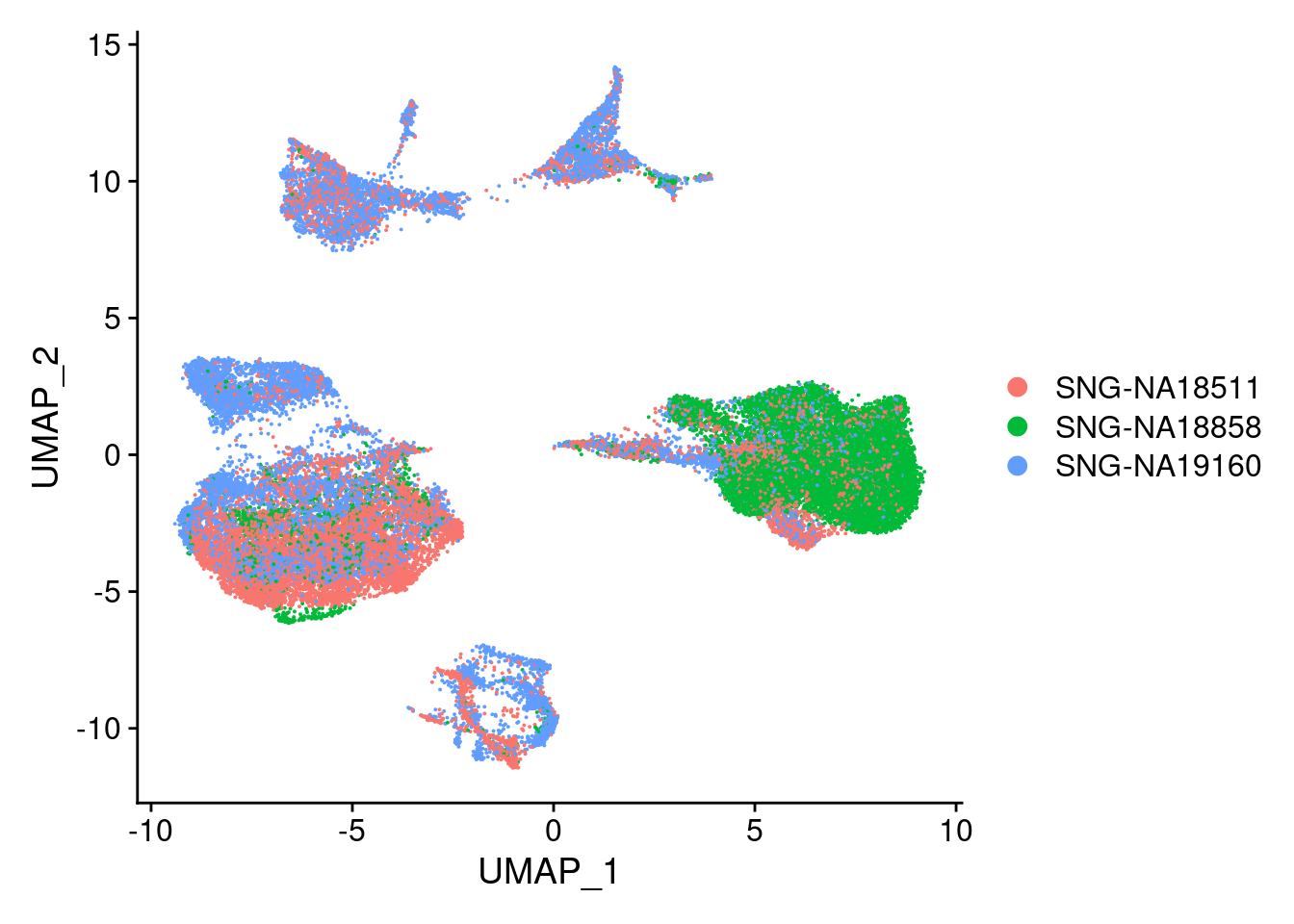
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
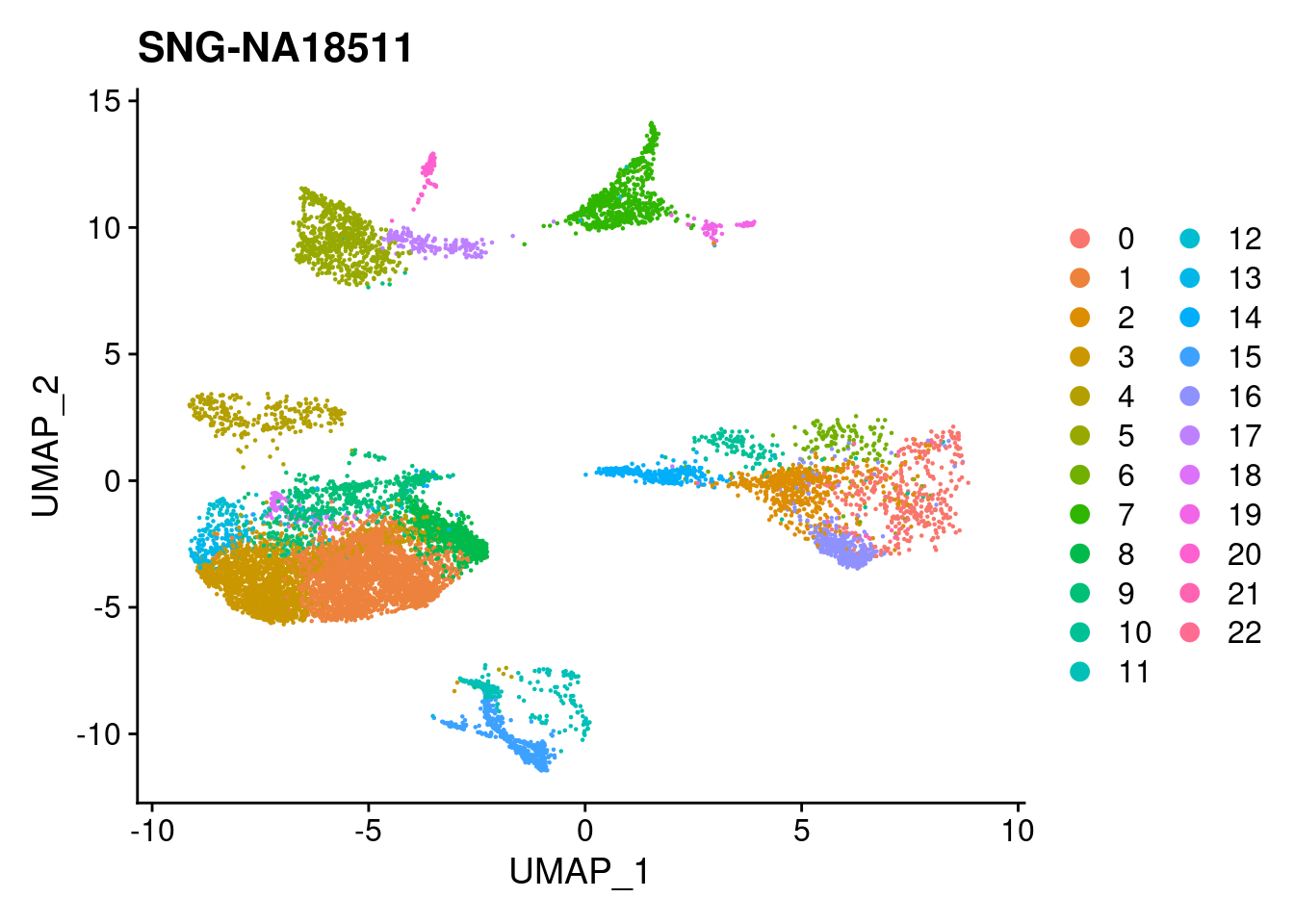
xlim <- c(min(merged@reductions$umap@cell.embeddings[,'UMAP_1']),
max(merged@reductions$umap@cell.embeddings[,'UMAP_1']))
ylim <- c(min(merged@reductions$umap@cell.embeddings[,'UMAP_2']),
max(merged@reductions$umap@cell.embeddings[,'UMAP_2']))
for (i in individuals)
{
print(DimPlot(merged, reduction = "umap",
cells = WhichCells(merged, expression = individual == i)) +
xlim(xlim) + ylim(ylim) + ggtitle(i))
}
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
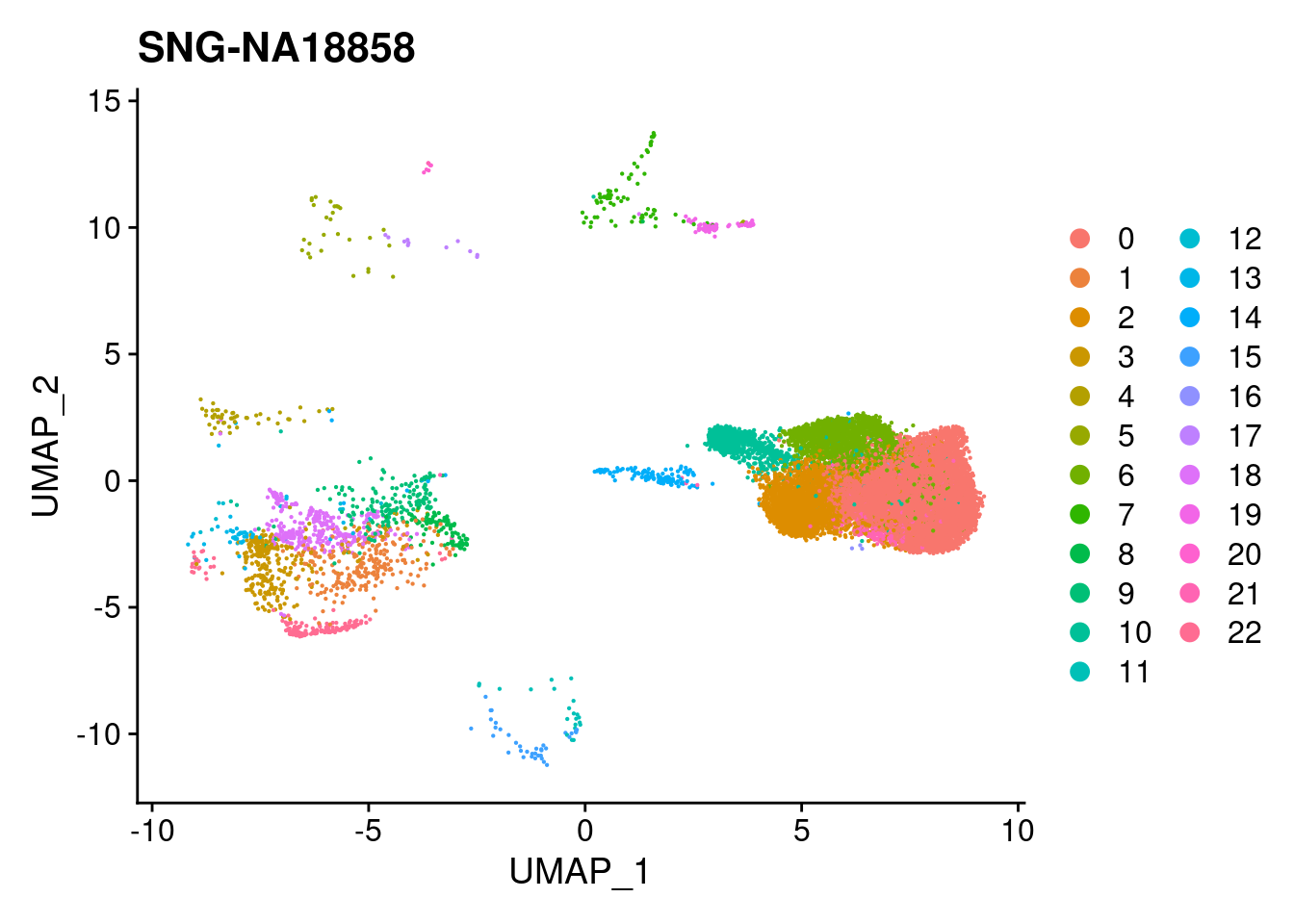
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
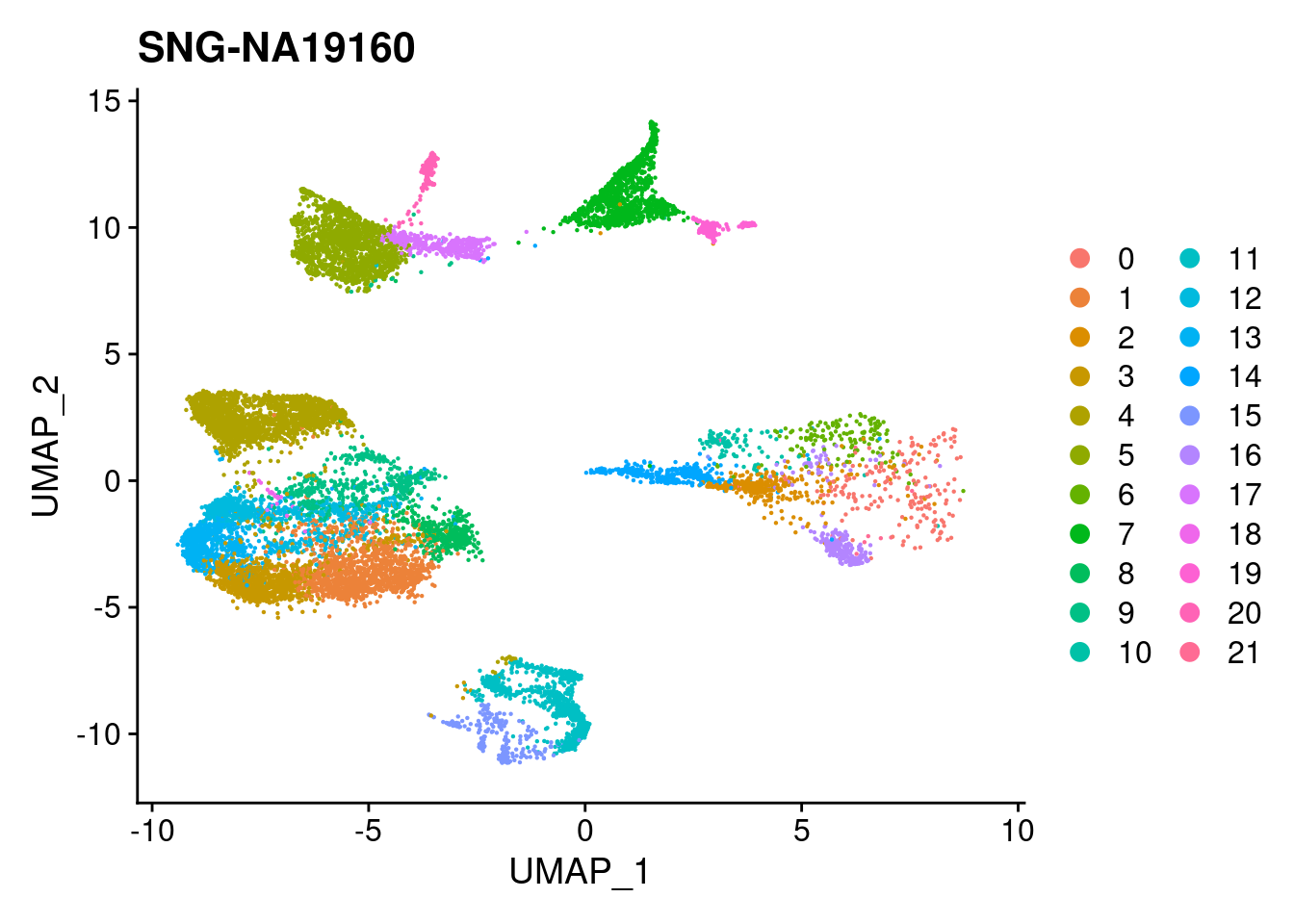
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#exploring similarity in the number of cells per individual between batches
merged.Batch1<- (subset(merged, Batch == "Batch1"))
b1t<- table(merged.Batch1$SCT_snn_res.0.8, merged.Batch1$individual)
remove("merged.Batch1")
b1tcolsums<- colSums(b1t)
percb1t<- b1t/b1tcolsums
merged.Batch2<- (subset(merged, Batch == "Batch2"))
b2t<- table(merged.Batch2$SCT_snn_res.0.8, merged.Batch2$individual)
remove("merged.Batch2")
b2tcolsums<- colSums(b2t)
percb2t<- b2t/b2tcolsums
merged.Batch3<- (subset(merged, Batch == "Batch3"))
b3t<- table(merged.Batch3$SCT_snn_res.0.8, merged.Batch3$individual)
remove("merged.Batch3")
b3tcolsums<- colSums(b3t)
percb3t<- b3t/b3tcolsumscols1<- c("Batch1_18511","Batch1_18858","Batch1_19160", "Batch2_18511", "Batch2_18858","Batch2_19160",
"Batch3_18511","Batch3_18858", "Batch3_19160")
cols2<- c("Batch1_18511", "Batch2_18511", "Batch3_18511","Batch1_18858", "Batch2_18858", "Batch3_18858","Batch1_19160", "Batch2_19160", "Batch3_19160")
fullpercs<- as.data.frame(cbind(percb1t[,1:3], percb2t,percb3t))
colnames(fullpercs)<-cols1
fullpercs<- cbind(fullpercs$Batch1_18511, fullpercs$Batch2_18511, fullpercs$Batch3_18511,
fullpercs$Batch1_18858, fullpercs$Batch2_18858, fullpercs$Batch3_18858,
fullpercs$Batch1_19160, fullpercs$Batch2_19160, fullpercs$Batch3_19160)
colnames(fullpercs)<-cols2
fullpercs_cor<- round(cor(fullpercs),2)
fullpercs_melt<- melt(fullpercs_cor)
ggplot(data= fullpercs_melt, aes(x=Var1, y=Var2, fill=value)) +
geom_tile(color="white") +
scale_fill_gradient2(low="blue", high="red", mid="white", midpoint = 0, limit= c(-1,1), space= "Lab", name="Pearson\nCorrelation") +
theme_minimal() +
ggtitle("Pairwise Pearson Correlation of the percent of cells from \neach cell line assigned to each Seurat Cluster\ncluster res. 0.8")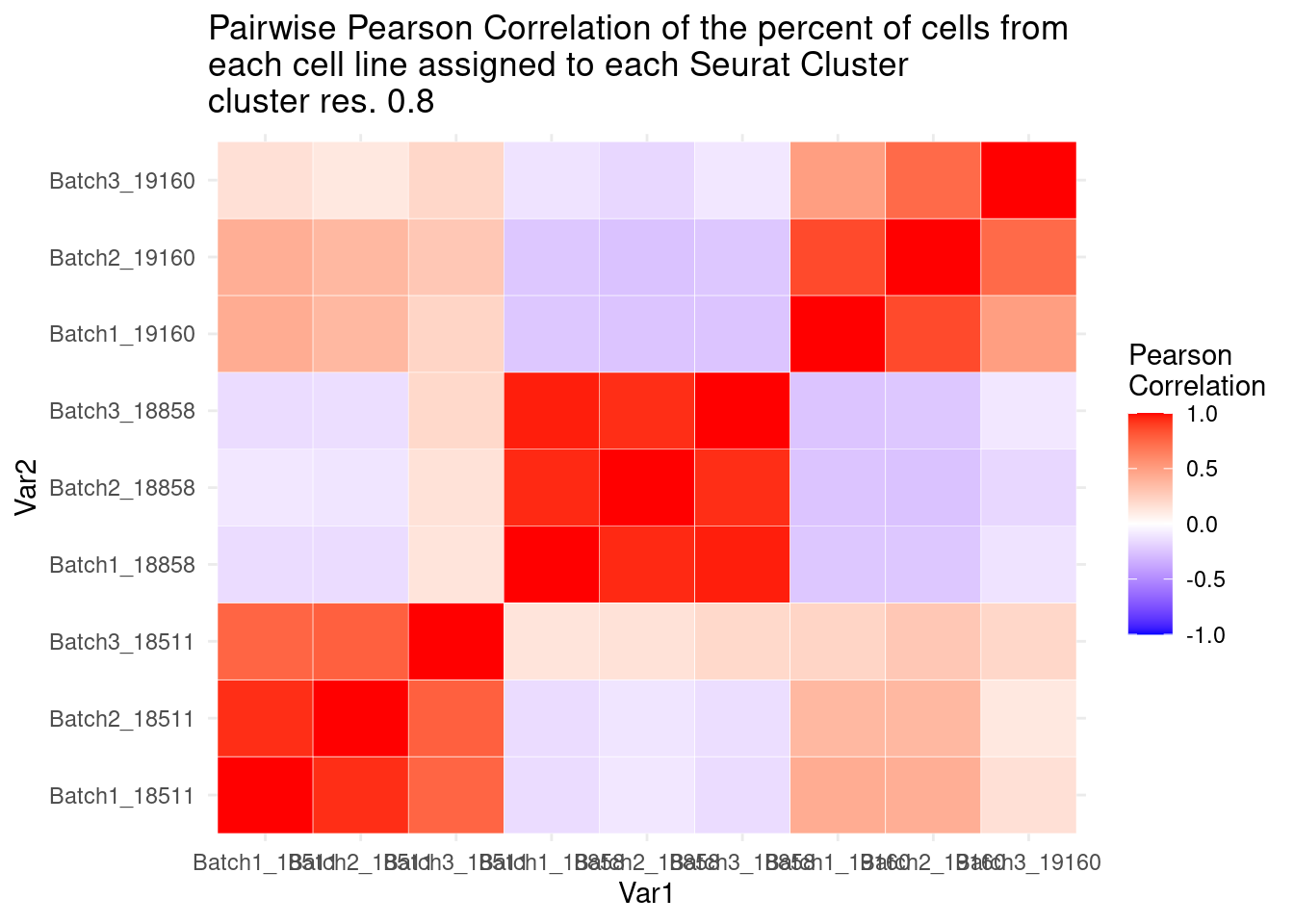
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#now clustering individual_Batch samples with hierarchical clustering/they will get reordered based on similarity
beauty<- colorRampPalette(brewer.pal(9,"Purples"))(200)
rownames(fullpercs)<- c(0:(nrow(fullpercs)-1))
heatmap(as.matrix(fullpercs), scale="none", col=beauty, cexCol = .7, cexRow=.6)
text(1:ncol(fullpercs),labels=names(fullpercs),srt=30)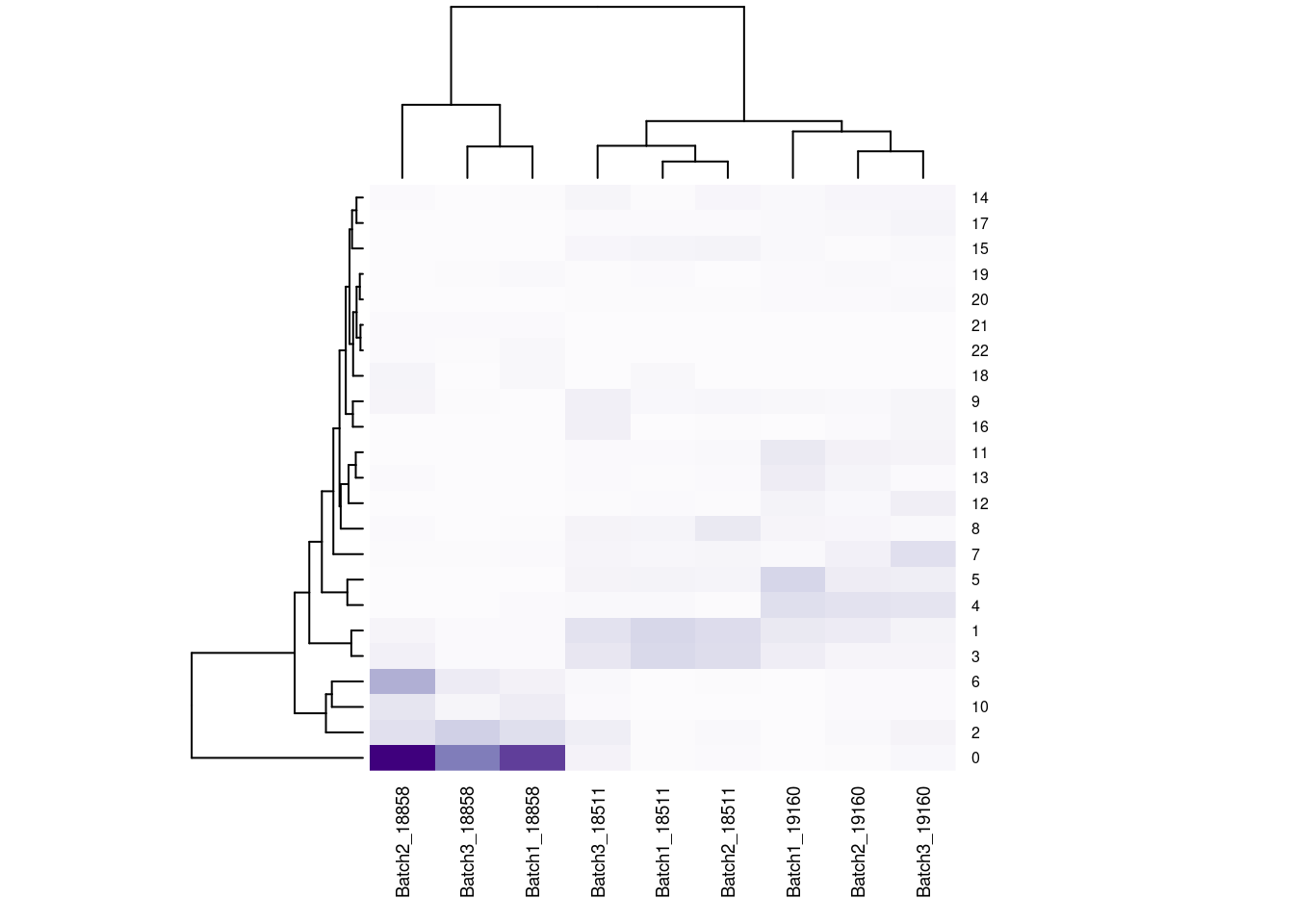
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
#generate a heatmap of the raw proportion of cells from each individual_batch in each seurat cluster. dendrograms based on similarity of the vectors. should be colored by the value(proportion), but some of the cluster/sample values to seem to match with the colorVlnPlot(merged, features= "percent.mt", group.by = "SCT_snn_res.1", pt.size = 0)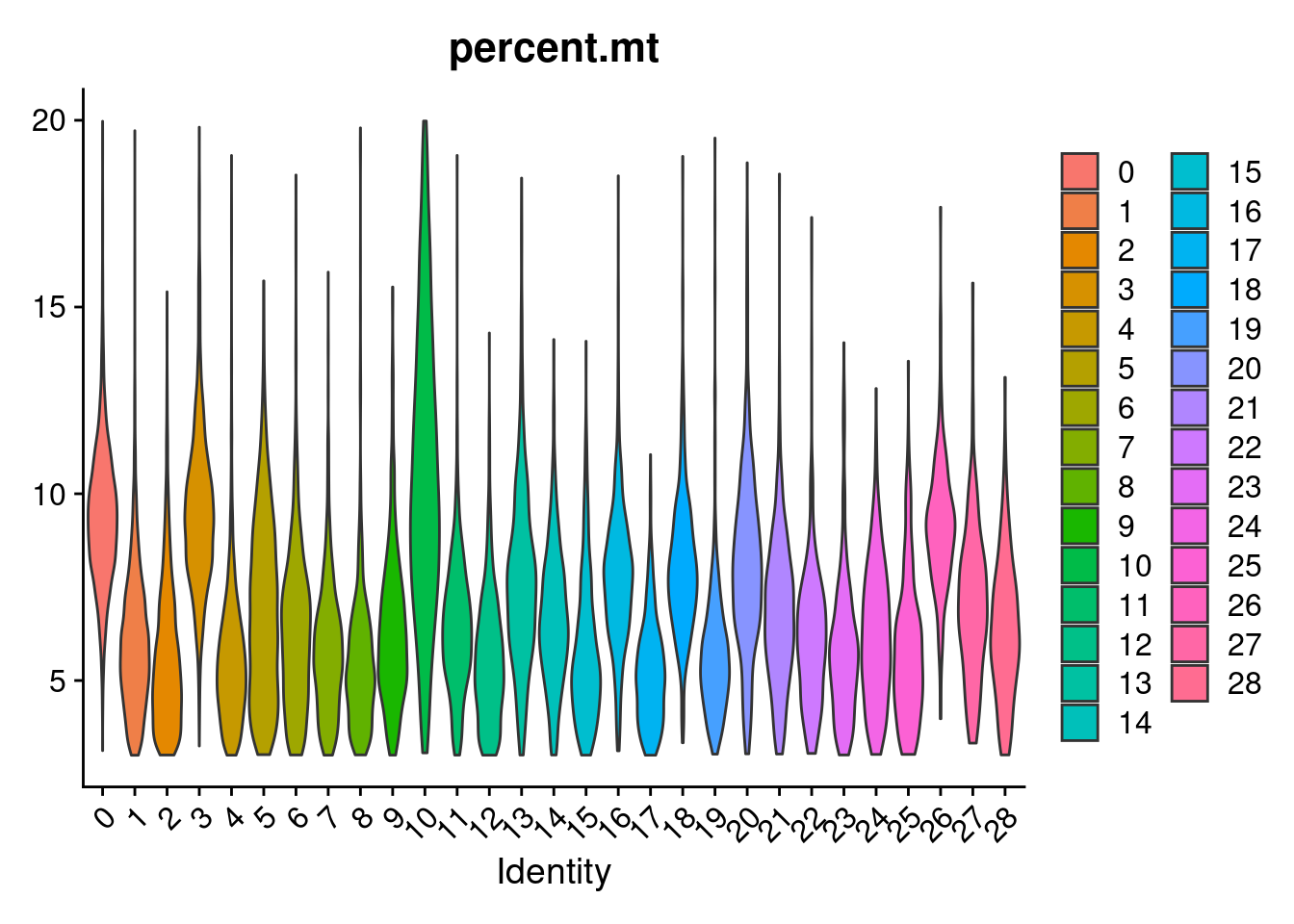
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
merged[["percent.rps"]]<- PercentageFeatureSet(merged, pattern = "^RPS")
merged[["percent.rpl"]]<- PercentageFeatureSet(merged, pattern = "^RPL")
merged[["percent.rp"]]<- merged[["percent.rps"]]+merged[["percent.rpl"]]
VlnPlot(merged, features= "percent.rp", group.by = "SCT_snn_res.1", pt.size=0)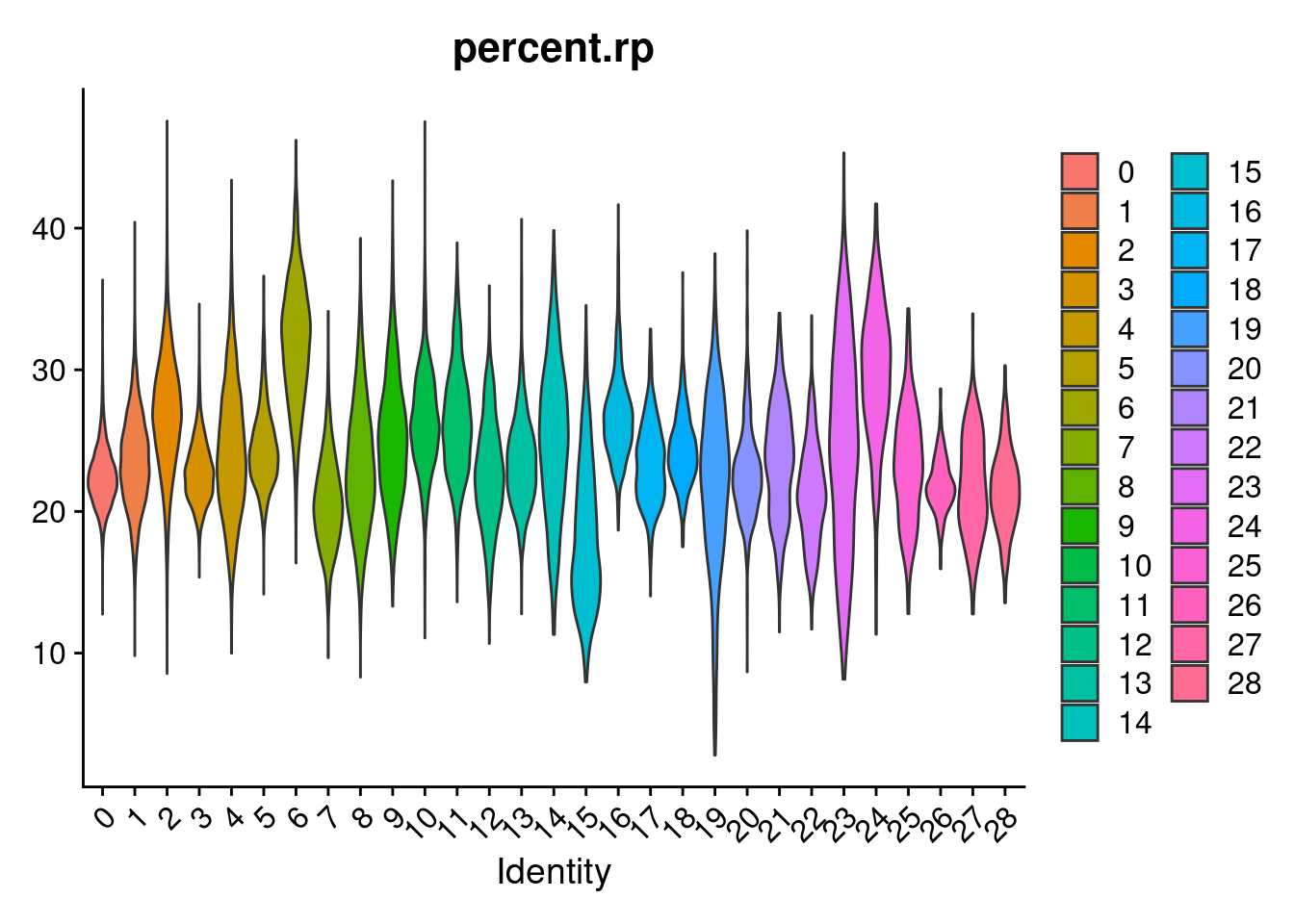
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
FeaturePlot(merged, features = "nFeature_RNA")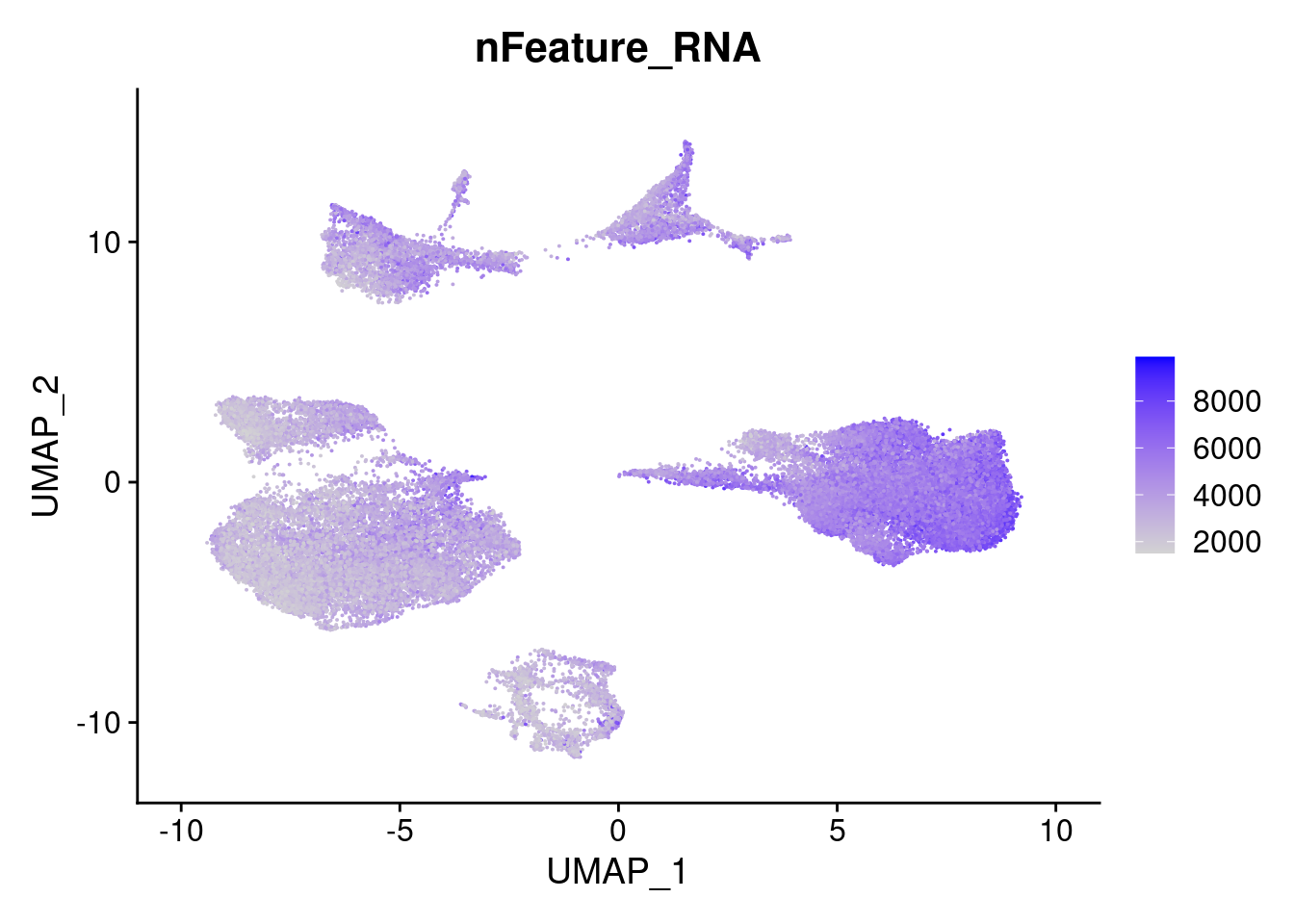
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
head(merged)An object of class Seurat
2 features across 42488 samples within 2 assays
Active assay: SCT (1 features, 1 variable features)
1 other assay present: RNA
3 dimensional reductions calculated: pca, harmony, umapVlnPlot(merged, features= "nFeature_RNA", group.by = "SCT_snn_res.1", pt.size=0)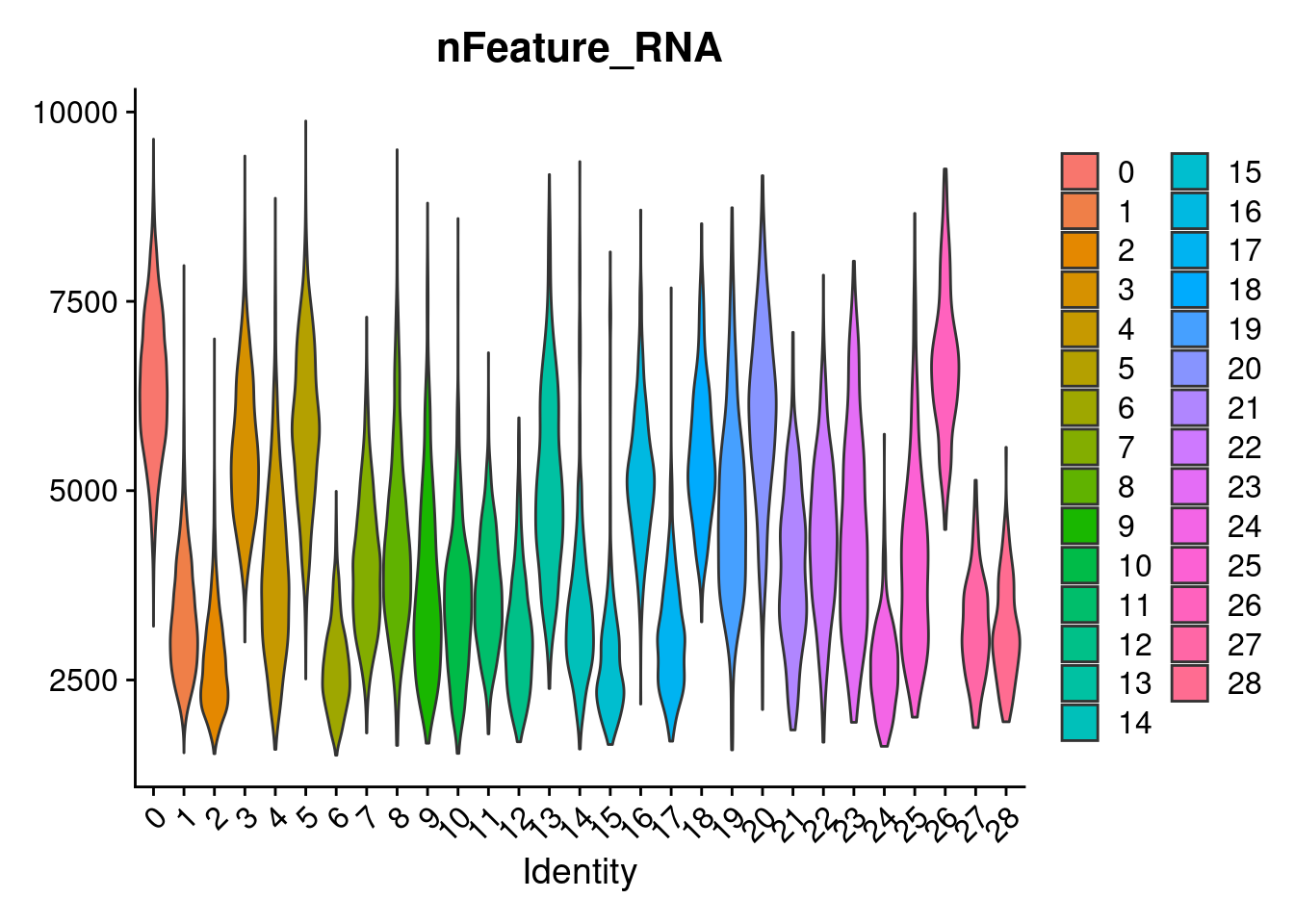
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
FeaturePlot(merged, features = c("POU5F1", "SOX17", "HAND1", "PAX6"), pt.size = 0.2, ncol=2, combine=T)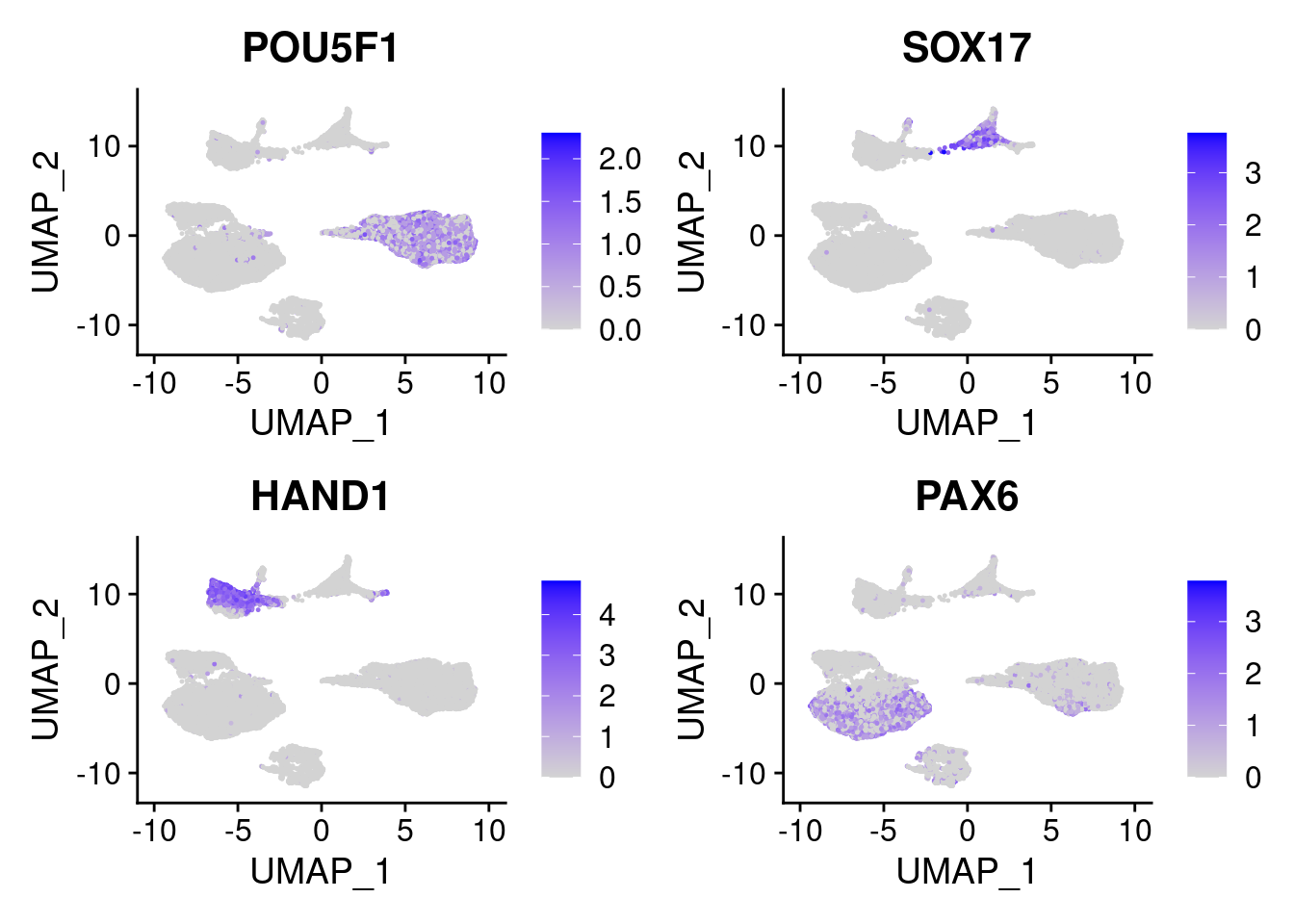
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
FeaturePlot(merged, features = c("FGB", "ECSCR", "NEUROD1", "SOX10"), pt.size = 0.2, ncol=2)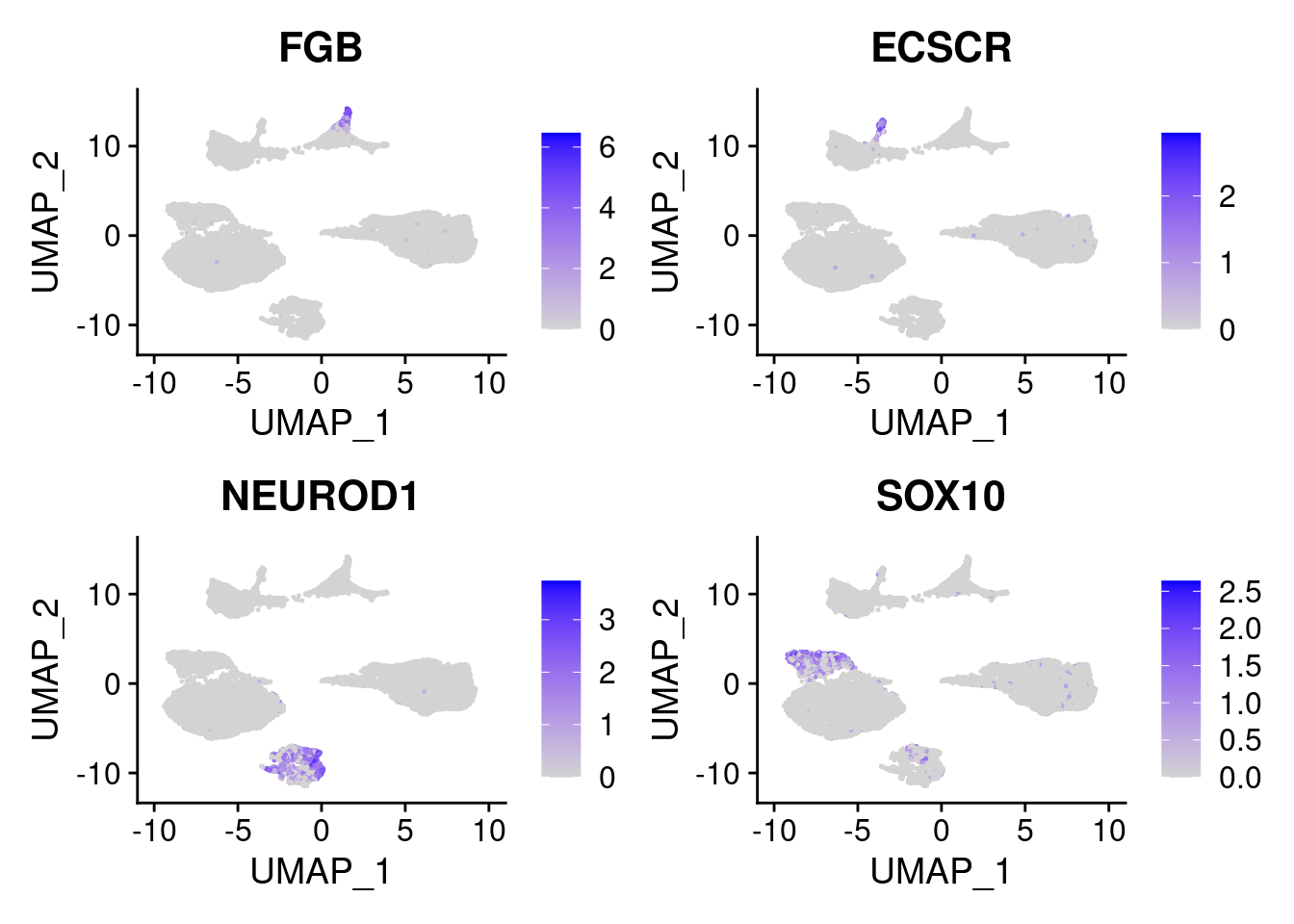
| Version | Author | Date |
|---|---|---|
| 421a225 | KLRhodes | 2020-08-10 |
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] reshape2_1.4.4 RColorBrewer_1.1-2 here_0.1-11 DataCombine_0.2.21
[5] ggplot2_3.3.2 harmony_1.0 Rcpp_1.0.5 Seurat_3.2.0
[9] workflowr_1.6.2
loaded via a namespace (and not attached):
[1] Rtsne_0.15 colorspace_1.4-1 deldir_0.1-28
[4] ellipsis_0.3.1 ggridges_0.5.2 rprojroot_1.3-2
[7] fs_1.4.2 spatstat.data_1.4-3 farver_2.0.3
[10] leiden_0.3.3 listenv_0.8.0 npsurv_0.4-0
[13] ggrepel_0.8.2 RSpectra_0.16-0 codetools_0.2-16
[16] splines_3.6.1 lsei_1.2-0 knitr_1.29
[19] polyclip_1.10-0 jsonlite_1.7.0 ica_1.0-2
[22] cluster_2.1.0 png_0.1-7 uwot_0.1.8
[25] shiny_1.5.0 sctransform_0.2.1 compiler_3.6.1
[28] httr_1.4.2 backports_1.1.8 Matrix_1.2-18
[31] fastmap_1.0.1 lazyeval_0.2.2 later_1.1.0.1
[34] htmltools_0.5.0 tools_3.6.1 rsvd_1.0.3
[37] igraph_1.2.5 gtable_0.3.0 glue_1.4.1
[40] RANN_2.6.1 dplyr_1.0.0 rappdirs_0.3.1
[43] spatstat_1.64-1 vctrs_0.3.2 gdata_2.18.0
[46] ape_5.3 nlme_3.1-140 lmtest_0.9-37
[49] xfun_0.16 stringr_1.4.0 globals_0.12.5
[52] mime_0.9 miniUI_0.1.1.1 lifecycle_0.2.0
[55] irlba_2.3.3 gtools_3.8.2 goftest_1.2-2
[58] future_1.18.0 MASS_7.3-51.4 zoo_1.8-8
[61] scales_1.1.1 promises_1.1.1 spatstat.utils_1.17-0
[64] parallel_3.6.1 yaml_2.2.1 reticulate_1.16
[67] pbapply_1.4-2 gridExtra_2.3 rpart_4.1-15
[70] stringi_1.4.6 caTools_1.18.0 rlang_0.4.7
[73] pkgconfig_2.0.3 bitops_1.0-6 evaluate_0.14
[76] lattice_0.20-38 ROCR_1.0-7 purrr_0.3.4
[79] tensor_1.5 labeling_0.3 patchwork_1.0.1
[82] htmlwidgets_1.5.1 cowplot_1.0.0 tidyselect_1.1.0
[85] RcppAnnoy_0.0.16 plyr_1.8.6 magrittr_1.5
[88] R6_2.4.1 gplots_3.0.4 generics_0.0.2
[91] withr_2.2.0 pillar_1.4.6 whisker_0.4
[94] mgcv_1.8-28 fitdistrplus_1.0-14 survival_3.2-3
[97] abind_1.4-5 tibble_3.0.3 future.apply_1.6.0
[100] crayon_1.3.4 KernSmooth_2.23-15 plotly_4.9.2.1
[103] rmarkdown_2.3 grid_3.6.1 data.table_1.13.0
[106] git2r_0.26.1 digest_0.6.25 xtable_1.8-4
[109] tidyr_1.1.0 httpuv_1.5.4 munsell_0.5.0
[112] viridisLite_0.3.0